当前位置:
X-MOL 学术
›
Luminescence
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Resonance Rayleigh scattering and spectrofluorimetric approaches for the selective determination of rupatadine using erythrosin B as a probe: application to content uniformity test
Luminescence ( IF 3.2 ) Pub Date : 2020-11-11 , DOI: 10.1002/bio.3983 Albandary Almahri 1 , Mohamed A Abdel-Lateef 2 , Ebtihal Samir 3 , Sayed M Derayea 4 , Mohamed A El Hamd 5, 6
Luminescence ( IF 3.2 ) Pub Date : 2020-11-11 , DOI: 10.1002/bio.3983 Albandary Almahri 1 , Mohamed A Abdel-Lateef 2 , Ebtihal Samir 3 , Sayed M Derayea 4 , Mohamed A El Hamd 5, 6
Affiliation
|
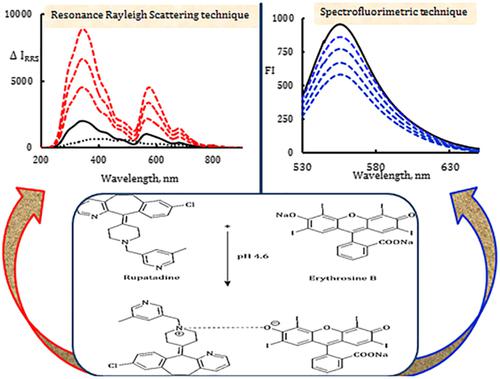
In this study, spectrofluorimetric and resonance Rayleigh scattering techniques were applied for the first time for determination of rupatadine through two validated methods. The proposed methods were based on a facile association complex formation between rupatadine and erythrosin B reagent in acidic medium. Spectrofluorimetric determination relied on the quenching effect of rupatadine on the fluorescence intensity of erythrosin B at 556 nm (excitation = 530 nm). Conversely, the resonance Rayleigh scattering (RRS) method relied on enhancement in the resonance Rayleigh scattering spectrum of erythrosin B at 344 nm after the addition of rupatadine. The developed methods produced linear results over ranges 0.15−2.0 μg/ml and 0.1−1.5 μg/ml, with detection limits of 0.030 μg/ml and 0.018 μg/ml for the spectrofluorimetric method and the RRS method, respectively. All reaction conditions for rupatadine–erythrosin B formation were optimized experimentally and both methods were validated according to International Council for Harmonisation guidelines. The developed methods were applied to estimate rupatadine content in its pharmaceutical tablet dosage form with acceptable recoveries. Furthermore, a content uniformity test for the commercial rupatadine tablets was successfully applied by the suggested spectroscopic methods according to United States Pharmacopeia guidelines.
中文翻译:

共振瑞利散射和荧光光谱法以赤藓红B为探针选择性测定卢帕他定:在含量均匀度测试中的应用
在这项研究中,光谱荧光和共振瑞利散射技术首次通过两种经过验证的方法用于测定卢帕他定。所提出的方法是基于在酸性介质中卢帕他定与赤藓红B试剂之间容易形成的缔合复合物。荧光光谱法的测定依赖于卢帕他定对556 nm(激发光= 530 nm)赤藓红B荧光强度的猝灭作用。相反,共振瑞利散射(RRS)方法依赖于添加卢帕他定后344 nm处赤藓红B的共振瑞利散射光谱的增强。在范围0.15线性结果产生的开发的方法- 2.0微克/毫升和0.1 -光谱荧光法和RRS方法的检出限分别为1.5μg/ ml和0.030μg/ ml和0.018μg/ ml。通过实验优化了所有用于形成雷帕他定-赤藓红B的反应条件,并根据国际协调委员会的指导原则对这两种方法进行了验证。所开发的方法被用于估计其药物片剂剂型中的卢帕他定含量,回收率可接受。此外,根据美国药典指南,通过建议的光谱方法已成功地对商品卢帕他定片进行了含量均一性测试。
更新日期:2020-11-11
中文翻译:

共振瑞利散射和荧光光谱法以赤藓红B为探针选择性测定卢帕他定:在含量均匀度测试中的应用
在这项研究中,光谱荧光和共振瑞利散射技术首次通过两种经过验证的方法用于测定卢帕他定。所提出的方法是基于在酸性介质中卢帕他定与赤藓红B试剂之间容易形成的缔合复合物。荧光光谱法的测定依赖于卢帕他定对556 nm(激发光= 530 nm)赤藓红B荧光强度的猝灭作用。相反,共振瑞利散射(RRS)方法依赖于添加卢帕他定后344 nm处赤藓红B的共振瑞利散射光谱的增强。在范围0.15线性结果产生的开发的方法- 2.0微克/毫升和0.1 -光谱荧光法和RRS方法的检出限分别为1.5μg/ ml和0.030μg/ ml和0.018μg/ ml。通过实验优化了所有用于形成雷帕他定-赤藓红B的反应条件,并根据国际协调委员会的指导原则对这两种方法进行了验证。所开发的方法被用于估计其药物片剂剂型中的卢帕他定含量,回收率可接受。此外,根据美国药典指南,通过建议的光谱方法已成功地对商品卢帕他定片进行了含量均一性测试。











































 京公网安备 11010802027423号
京公网安备 11010802027423号