当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diagnostic characterization of respiratory allergies by means of a multiplex immunoassay
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-11-11 , DOI: 10.1111/cei.13548 J L M Millen 1, 2 , I Willems 1, 2 , G Slingers 1, 3, 4 , M Raes 3, 4 , G Koppen 1 , S A S Langie 1, 2, 5
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-11-11 , DOI: 10.1111/cei.13548 J L M Millen 1, 2 , I Willems 1, 2 , G Slingers 1, 3, 4 , M Raes 3, 4 , G Koppen 1 , S A S Langie 1, 2, 5
Affiliation
|
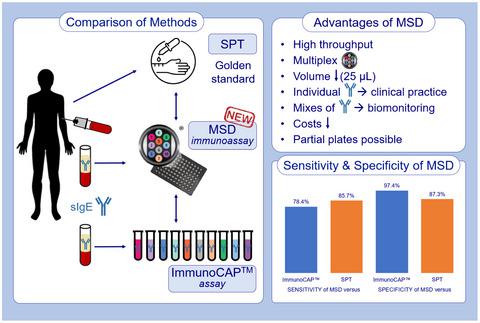
Allergic sensitization is commonly assessed in patients by performing the skin prick test (SPT) or determining specific immunoglobulin (IgE) levels in blood samples with the ImmunoCAP™ assay, which measures each allergen and sample separately. This paper explores the possibility to investigate respiratory allergies with a high throughput method, the Meso Scale Discovery (MSD) multiplex immunoassay, measuring IgE levels in low volumes of blood. The MSD multiplex immunoassay, developed and optimized with standards and allergens from Radim Diagnostics, was validated against the SPT and the ImmunoCAP assay. For 18 adults (15 respiratory allergy patients and three controls), blood collection and the SPT were performed within the same hour. Pearson correlations and Bland–Altman analysis showed high comparability of the MSD multiplex immunoassay with the SPT and the ImmunoCAP assay, except for house dust mite. The sensitivity of the MSD multiplexed assay was ≥78% for most allergens compared to the SPT and ImmunoCAP assay. Additionally, the specificity of the MSD multiplex immunoassay was ≥ 87% – the majority showing 100% specificity. Only the rye allergen had a low specificity when compared to the SPT, probably due to cross‐reactivity. The reproducibility of the MSD multiplex immunoassay, assessed as intra‐ and interassay reproducibility and biological variability between different sampling moments, showed significantly high correlations (r = 0·943–1) for all tested subjects (apart from subject 13; r = 0·65–0·99). The MSD multiplex immunoassay is a reliable method to detect specific IgE levels against respiratory allergens in a multiplexed and high‐throughput manner, using blood samples as small as from a finger prick.
中文翻译:

通过多重免疫分析对呼吸道过敏症进行诊断表征
通常通过执行皮肤点刺试验 (SPT) 或使用 ImmunoCAP™ 测定法确定血液样本中的特定免疫球蛋白 (IgE) 水平来评估患者的过敏反应,该测定法分别测量每个过敏原和样本。本文探讨了使用高通量方法研究呼吸道过敏的可能性,即 Meso Scale Discovery (MSD) 多重免疫分析,测量少量血液中的 IgE 水平。使用 Radim Diagnostics 的标准品和过敏原开发和优化的 MSD 多重免疫测定已针对 SPT 和 ImmunoCAP 测定进行了验证。对于 18 名成人(15 名呼吸系统过敏患者和 3 名对照者),在同一小时内进行了采血和 SPT。Pearson 相关性和 Bland-Altman 分析表明 MSD 多重免疫测定与 SPT 和 ImmunoCAP 测定具有高度可比性,但屋尘螨除外。与 SPT 和 ImmunoCAP 检测相比,MSD 多重检测对大多数过敏原的敏感性≥78%。此外,MSD 多重免疫测定的特异性≥ 87% – 大多数表现出 100% 的特异性。与 SPT 相比,只有黑麦过敏原的特异性较低,这可能是由于交叉反应。MSD 多重免疫测定的再现性,评估为不同采样时刻之间的测定内和测定间再现性和生物学变异性,显示出显着的高相关性。与 SPT 和 ImmunoCAP 检测相比,MSD 多重检测对大多数过敏原的敏感性≥78%。此外,MSD 多重免疫测定的特异性≥ 87% – 大多数表现出 100% 的特异性。与 SPT 相比,只有黑麦过敏原的特异性较低,这可能是由于交叉反应。MSD 多重免疫测定的再现性,评估为不同采样时刻之间的测定内和测定间再现性和生物学变异性,显示出显着的高相关性。与 SPT 和 ImmunoCAP 检测相比,MSD 多重检测对大多数过敏原的敏感性≥78%。此外,MSD 多重免疫测定的特异性≥ 87% – 大多数表现出 100% 的特异性。与 SPT 相比,只有黑麦过敏原的特异性较低,这可能是由于交叉反应。MSD 多重免疫测定的再现性,评估为不同采样时刻之间的测定内和测定间再现性和生物学变异性,显示出显着的高相关性。r = 0·943-1)对于所有测试对象(除了对象 13;r = 0·65-0·99)。MSD 多重免疫分析是一种可靠的方法,可以以多重和高通量的方式检测针对呼吸道过敏原的特定 IgE 水平,使用的血液样本小到手指刺痛。
更新日期:2021-01-14
中文翻译:

通过多重免疫分析对呼吸道过敏症进行诊断表征
通常通过执行皮肤点刺试验 (SPT) 或使用 ImmunoCAP™ 测定法确定血液样本中的特定免疫球蛋白 (IgE) 水平来评估患者的过敏反应,该测定法分别测量每个过敏原和样本。本文探讨了使用高通量方法研究呼吸道过敏的可能性,即 Meso Scale Discovery (MSD) 多重免疫分析,测量少量血液中的 IgE 水平。使用 Radim Diagnostics 的标准品和过敏原开发和优化的 MSD 多重免疫测定已针对 SPT 和 ImmunoCAP 测定进行了验证。对于 18 名成人(15 名呼吸系统过敏患者和 3 名对照者),在同一小时内进行了采血和 SPT。Pearson 相关性和 Bland-Altman 分析表明 MSD 多重免疫测定与 SPT 和 ImmunoCAP 测定具有高度可比性,但屋尘螨除外。与 SPT 和 ImmunoCAP 检测相比,MSD 多重检测对大多数过敏原的敏感性≥78%。此外,MSD 多重免疫测定的特异性≥ 87% – 大多数表现出 100% 的特异性。与 SPT 相比,只有黑麦过敏原的特异性较低,这可能是由于交叉反应。MSD 多重免疫测定的再现性,评估为不同采样时刻之间的测定内和测定间再现性和生物学变异性,显示出显着的高相关性。与 SPT 和 ImmunoCAP 检测相比,MSD 多重检测对大多数过敏原的敏感性≥78%。此外,MSD 多重免疫测定的特异性≥ 87% – 大多数表现出 100% 的特异性。与 SPT 相比,只有黑麦过敏原的特异性较低,这可能是由于交叉反应。MSD 多重免疫测定的再现性,评估为不同采样时刻之间的测定内和测定间再现性和生物学变异性,显示出显着的高相关性。与 SPT 和 ImmunoCAP 检测相比,MSD 多重检测对大多数过敏原的敏感性≥78%。此外,MSD 多重免疫测定的特异性≥ 87% – 大多数表现出 100% 的特异性。与 SPT 相比,只有黑麦过敏原的特异性较低,这可能是由于交叉反应。MSD 多重免疫测定的再现性,评估为不同采样时刻之间的测定内和测定间再现性和生物学变异性,显示出显着的高相关性。r = 0·943-1)对于所有测试对象(除了对象 13;r = 0·65-0·99)。MSD 多重免疫分析是一种可靠的方法,可以以多重和高通量的方式检测针对呼吸道过敏原的特定 IgE 水平,使用的血液样本小到手指刺痛。











































 京公网安备 11010802027423号
京公网安备 11010802027423号