当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,3‐Dipolar Cycloaddition between Dehydroalanines and C,N‐Cyclic Azomethine Imines: Application to Late‐Stage Peptide Modification
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-12 , DOI: 10.1002/anie.202012523 Guangjun Bao 1 , Peng Wang 1 , Guofeng Li 2 , Changjun Yu 1 , Yiping Li 1 , Yuyang Liu 1 , Zeyuan He 1 , Tiantian Zhao 1 , Jing Rao 1 , Junqiu Xie 1 , Liang Hong 2 , Wangsheng Sun 1 , Rui Wang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-12 , DOI: 10.1002/anie.202012523 Guangjun Bao 1 , Peng Wang 1 , Guofeng Li 2 , Changjun Yu 1 , Yiping Li 1 , Yuyang Liu 1 , Zeyuan He 1 , Tiantian Zhao 1 , Jing Rao 1 , Junqiu Xie 1 , Liang Hong 2 , Wangsheng Sun 1 , Rui Wang 1
Affiliation
|
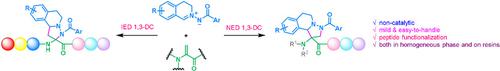
A non‐catalytic, mild, and easy‐to‐handle protecting group switched 1,3‐dipolar cycloaddition (1,3‐DC) between bi‐ or mono‐N‐protected Dha and C,N‐cyclic azomethine imines, which afford various quaternary amino acids with diverse scaffolds, is disclosed. Specifically, normal‐electron‐demand 1,3‐DC reaction occurs between bi‐N‐protected Dha and C,N‐cyclic azomethine imines, while inverse‐electron‐demand 1,3‐DC reaction occurs between mono‐N‐protected Dha and C,N‐cyclic azomethine imines. Above all, the reactions can be carried out between peptides with Dha residues at the position of interest and C,N‐cyclic azomethine imines, both in homogeneous phase and on resins in SPPS. It provides a new toolkit for late‐stage peptide modification, labeling, and peptide–drug conjugation. To shed light on the high regioselectivity of the reaction, DFT calculations were carried out, which were qualitatively consistent with the experimental observations.
中文翻译:

脱氢丙氨酸与C,N-环偶氮甲亚胺之间的1,3-偶极环加成:在后期肽修饰中的应用
非催化,温和且易于操作的保护基在双或单N-保护的Dha与C,N-环偶氮甲亚胺之间切换1,3-偶极环加成(1,3-DC)公开了具有各种支架的各种季氨基酸。具体来说,在双N保护的Dha与C,N环甲亚胺亚胺之间发生正常的电子需求1,3-DC反应,而在单N保护的Dha之间发生反电子需求的1,3-DC反应。和C,N-环偶氮甲亚胺。最重要的是,该反应可以在目标位置具有Dha残基的肽与C,N-环偶氮甲亚胺在均相和SPPS树脂上进行。它为后期肽修饰,标记和肽-药物结合提供了一个新的工具包。为了阐明反应的高区域选择性,
更新日期:2020-11-12
中文翻译:

脱氢丙氨酸与C,N-环偶氮甲亚胺之间的1,3-偶极环加成:在后期肽修饰中的应用
非催化,温和且易于操作的保护基在双或单N-保护的Dha与C,N-环偶氮甲亚胺之间切换1,3-偶极环加成(1,3-DC)公开了具有各种支架的各种季氨基酸。具体来说,在双N保护的Dha与C,N环甲亚胺亚胺之间发生正常的电子需求1,3-DC反应,而在单N保护的Dha之间发生反电子需求的1,3-DC反应。和C,N-环偶氮甲亚胺。最重要的是,该反应可以在目标位置具有Dha残基的肽与C,N-环偶氮甲亚胺在均相和SPPS树脂上进行。它为后期肽修饰,标记和肽-药物结合提供了一个新的工具包。为了阐明反应的高区域选择性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号