Water Research ( IF 11.4 ) Pub Date : 2020-11-12 , DOI: 10.1016/j.watres.2020.116632 Yuan Zhuang , Congcong Shen , Yifan Gu , Ruya Chen , Baoyou Shi
|
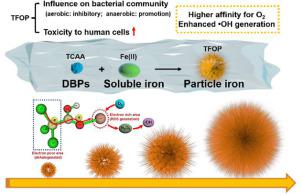
In drinking water distribution system (DWDS), disinfection byproducts (DBPs) have a large possibility of participating in iron oxidation by dissolved oxygen (DO), which may induce particle structure transformations and increase unknown risks. In this work, the influence of trichloroacetic acid (TCAA, one of the most typical DBPs) on iron oxidation processes was studied, and the potential effects of the resulting α-FeOOH particles were evaluated through two aspects: (i) influence on the bacterial community and (ii) toxicity to human cells. TCAA promoted iron oxidation process through an Fe−O−C linkage, which led to a sharper surface of the particles (TCAA-mediated Fe oxide particles, TFOP) than that without TCAA (Fe oxide particles, FOP). Interestingly, the influence of particles on the richness of bacterial community of drinking water was different under anaerobic and aerobic conditions: under anaerobic conditions, the richness of bacterial community increased with the addition of particles, while under aerobic conditions, the richness of bacterial community decreased. The higher affinity of TFOP for electron-accepting DO than FOP indicated the role of DO on TFOP under aerobic conditions. TFOP exhibited the strongest cytotoxicity among FOP and the actual deposits. DFT calculations confirmed that TCAA in iron particles promoted the adsorption and dissociation of H2O2 to generate more •OH with an obvious decrease in the energy barrier from 1.51 to 0.80 eV. This study indicates the high potential of adverse effects of DBPs on loose deposits in DWDS and gives implications for the control of DBPs and deposits in drinking water.
中文翻译:

三氯乙酸对铁氧化的影响:对控制饮用水中DBP和沉积物的意义
在饮用水分配系统(DWDS)中,消毒副产物(DBP)很有可能通过溶解氧(DO)参与铁氧化,这可能会引起颗粒结构转变并增加未知风险。在这项工作中,研究了三氯乙酸(TCAA,最典型的DBP之一)对铁氧化过程的影响,并从两个方面评估了生成的α-FeOOH颗粒的潜在影响:(i)对细菌的影响社区和(ii)对人体细胞的毒性。TCAA通过Fe-O-C键促进了铁的氧化过程,与没有TCAA的颗粒(Fe氧化物颗粒,FOP)相比,TCAA的颗粒表面更为尖锐(TCAA介导的Fe氧化物颗粒,TFOP)。有趣的是 在厌氧和好氧条件下,颗粒对饮用水细菌群落丰富度的影响是不同的:在厌氧条件下,随着颗粒的添加细菌群落的丰富度增加,而在有氧条件下,细菌群落的丰富度降低。TFOP对电子接受DO的亲和力高于FOP,表明在有氧条件下DO对TFOP的作用。TFOP在FOP和实际沉积物中表现出最强的细胞毒性。DFT计算证实铁颗粒中的TCAA促进了H的吸附和解离 TFOP对电子接受DO的亲和力高于FOP,表明在有氧条件下DO对TFOP的作用。TFOP在FOP和实际沉积物中表现出最强的细胞毒性。DFT计算证实铁颗粒中的TCAA促进了H的吸附和解离 TFOP对电子接受DO的亲和力高于FOP,表明在有氧条件下DO对TFOP的作用。TFOP在FOP和实际沉积物中表现出最强的细胞毒性。DFT计算证实铁颗粒中的TCAA促进了H的吸附和解离2 O 2生成更多的•OH,并且能垒从1.51 eV明显降低到0.80 eV。这项研究表明,DBP对DWDS中的松散沉积物产生不良影响的可能性很高,并且对控制DBP和饮用水中的沉积物具有重要意义。









































 京公网安备 11010802027423号
京公网安备 11010802027423号