当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The enthalpies of dilution of some dipeptides in water at 298.15 K
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.jct.2020.106338 Sylwia Belica-Pacha , Martyna Michalska-Tężycka , Magdalena Ciechańska , Artur Stępniak , Bartłomiej Pałecz
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.jct.2020.106338 Sylwia Belica-Pacha , Martyna Michalska-Tężycka , Magdalena Ciechańska , Artur Stępniak , Bartłomiej Pałecz
|
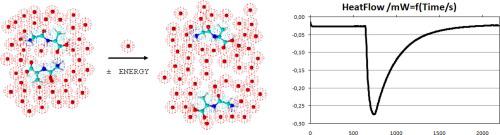
Abstract The enthalpies of dilution of glycyl-L-α-alanine, glycyl-L-α-leucine and glycyl-L-glutamine have been measured in water at T= 298.15 K. The experimental results were used to calculate the enthalpic coefficients of the interactions between dipeptides molecules in water based on McMillan–Mayer's model. The values of the interaction parameters were interpreted in terms of the hydrophobic or hydrophilic properties of the amino acid side chins in the examined dipeptide molecules and the influence on their interactions with each other in water.
中文翻译:

某些二肽在 298.15 K 时在水中的稀释焓
摘要 测定了甘氨酰-L-α-丙氨酸、甘氨酰-L-α-亮氨酸和甘氨酰-L-谷氨酰胺在 T=298.15 K 的水中的稀释焓。基于 McMillan-Mayer 模型的水中二肽分子之间的相互作用。相互作用参数的值根据所检查的二肽分子中氨基酸侧下巴的疏水或亲水特性以及对它们在水中彼此相互作用的影响来解释。
更新日期:2021-03-01
中文翻译:

某些二肽在 298.15 K 时在水中的稀释焓
摘要 测定了甘氨酰-L-α-丙氨酸、甘氨酰-L-α-亮氨酸和甘氨酰-L-谷氨酰胺在 T=298.15 K 的水中的稀释焓。基于 McMillan-Mayer 模型的水中二肽分子之间的相互作用。相互作用参数的值根据所检查的二肽分子中氨基酸侧下巴的疏水或亲水特性以及对它们在水中彼此相互作用的影响来解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号