Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2020-11-12 , DOI: 10.1016/j.jcou.2020.101351 Alejandro Bermejo-López , Beñat Pereda-Ayo , José A. González-Marcos , Juan R. González-Velasco
|
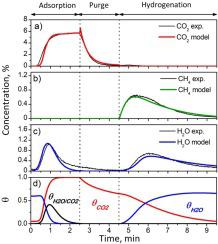
A dynamic kinetic model is proposed to describe the CO2 adsorption and in situ hydrogenation to CH4 on a 4%Ru-10%Na2CO3/Al2O3 catalyst. One dimensional isothermal heterogeneous plug flow reactor model with axial dispersion is considered. The reaction network and kinetic equations are deduced from experimental data analysis. The molecular modeling includes competitive adsorption of CO2 and H2O on same adsorption site. During CO2 storage period, CO2 is adsorbed onto Na2O species to form Na2CO3. Alternatively, CO2 can be also adsorbed onto NaOH species releasing H2O to the gas phase, which can further be adsorbed onto free adsorption sites located downstream. It was needed to consider formation of unstable bicarbonates to fit the experimental data. During CO2 hydrogenation step, adsorbed carbonates are decomposed, promoted by the presence of H2. CH4 is then formed through the Sabatier’s reaction and the as-formed H2O interacts with regenerated adsorption sites. Temporal evolution of CO2, CH4 and H2O during CO2 storage and hydrogenation cycles is accurately predicted by the model, for a wide range of reactant concentrations and temperature (250−400 °C), gaining understanding on mechanisms and dynamics of the process on dual function materials.
中文翻译:

在双功能Ru-Na 2 CO 3 / Al 2 O 3催化剂上模拟CO 2的捕获并原位转化为CH 4
提出了一个动力学模型来描述在4%Ru-10%Na 2 CO 3 / Al 2 O 3催化剂上CO 2的吸附和原位加氢成CH 4。考虑轴向分散的一维等温非均质塞流反应器模型。从实验数据分析推导了反应网络和动力学方程。分子模拟包括CO的竞争吸附2和H 2 O于相同的吸附位点。在CO 2储存期间,CO 2吸附到Na 2 O物种上形成Na 2 CO 3。或者,CO 2也可以被吸附到NaOH物质上,从而将H 2 O释放到气相中,而H 2 O可以被进一步吸附到位于下游的自由吸附点上。需要考虑形成不稳定的碳酸氢盐以适应实验数据。在CO 2加氢步骤中,H 2的存在促进了吸附的碳酸盐分解。然后通过Sabatier反应形成CH 4,而形成的H 2 O与再生的吸附位相互作用。CO 2期间CO 2,CH 4和H 2 O的时间演变 该模型可在广泛的反应物浓度和温度(250-400°C)范围内,通过模型准确地预测储存和氢化周期,从而加深对双功能材料工艺过程的机理和动力学的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号