当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polymerization Mechanism of 1,3-benzoxazine Catalyzed by PCl5 and Rearrangement of Chemical Structures
European Polymer Journal ( IF 5.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.eurpolymj.2020.110133 Shuai Zhang , Qichao Ran , Yi Gu
European Polymer Journal ( IF 5.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.eurpolymj.2020.110133 Shuai Zhang , Qichao Ran , Yi Gu
|
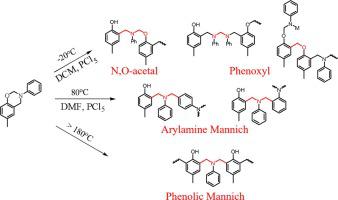
Abstract Ring-opening polymerization of benzoxazine monomers is a complex process and various chemical structures including N,O-acetal structures, phenolic Mannich structures and arylamine Mannich structures are formed in polybenzoxazines. To understand the polymerization mechanism, the effects of temperature, time and solvent polarity on the polymerization routes, chemical structures and thermal properties were studied. It was discovered that the N,O-acetal structures and the phenoxy structures can be obtained in a low-polarity solvent like dichloromethane at low temperature (-20 oC) with the aid of PCl5, while the arylamine Mannich structures can be readily generated in polar solvent like N,N-Dimethylformamide at high temperature (>80 oC) in the presence of PCl5. However, the phenolic Mannich structures can be directly formed at high temperature (>180 oC) without any catalysts. Upon prolonging the reaction time or elevating the temperature, the phenoxy structures easily rearranged into the N,O-acetal structures or the arylamine Mannich structures. Further increasing the temperature will cause the arylamine Mannich structures to rearrange into the phenolic Mannich structures and even the phenolic methylene structures. Therefore, both phenoxy structures and N,O-acetal structures showed poor thermal stability; while the arylamine Mannich structures possessed lower initial decomposition temperature but higher char yield compared with the phenolic Mannich structures because of the formation of thermally unstable iminium ions and the anchoring of dangling aniline moieties.
中文翻译:

PCl5催化1,3-苯并恶嗪聚合机理及化学结构重排
摘要 苯并恶嗪单体的开环聚合是一个复杂的过程,在聚苯并恶嗪中形成了多种化学结构,包括N,O-缩醛结构、酚类曼尼希结构和芳胺曼尼希结构。为了理解聚合机理,研究了温度、时间和溶剂极性对聚合路线、化学结构和热性能的影响。发现N,O-缩醛结构和苯氧基结构可以在低极性溶剂如二氯甲烷中在低温(-20 oC)下借助PCl5获得,而芳胺曼尼希结构可以很容易地在极性溶剂,如 N,N-二甲基甲酰胺,在高温 (>80 oC) 和 PCl5 存在下。然而,酚类曼尼希结构可以在高温(> 180 oC)下直接形成,无需任何催化剂。在延长反应时间或升高温度后,苯氧基结构很容易重排成 N,O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。在延长反应时间或升高温度后,苯氧基结构很容易重排成 N,O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。在延长反应时间或升高温度后,苯氧基结构很容易重排成 N,O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。
更新日期:2021-01-01
中文翻译:

PCl5催化1,3-苯并恶嗪聚合机理及化学结构重排
摘要 苯并恶嗪单体的开环聚合是一个复杂的过程,在聚苯并恶嗪中形成了多种化学结构,包括N,O-缩醛结构、酚类曼尼希结构和芳胺曼尼希结构。为了理解聚合机理,研究了温度、时间和溶剂极性对聚合路线、化学结构和热性能的影响。发现N,O-缩醛结构和苯氧基结构可以在低极性溶剂如二氯甲烷中在低温(-20 oC)下借助PCl5获得,而芳胺曼尼希结构可以很容易地在极性溶剂,如 N,N-二甲基甲酰胺,在高温 (>80 oC) 和 PCl5 存在下。然而,酚类曼尼希结构可以在高温(> 180 oC)下直接形成,无需任何催化剂。在延长反应时间或升高温度后,苯氧基结构很容易重排成 N,O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。在延长反应时间或升高温度后,苯氧基结构很容易重排成 N,O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。在延长反应时间或升高温度后,苯氧基结构很容易重排成 N,O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。O-缩醛结构或芳胺曼尼希结构。进一步升高温度将导致芳胺曼尼希结构重排为酚类曼尼希结构甚至酚类亚甲基结构。因此,苯氧基结构和N,O-缩醛结构均表现出较差的热稳定性;与酚类曼尼希结构相比,芳胺曼尼希结构具有较低的初始分解温度,但由于形成了热不稳定的亚胺离子和悬空苯胺部分的锚定,因此具有较高的焦炭产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号