Current Opinion in Cell Biology ( IF 6.0 ) Pub Date : 2020-11-12 , DOI: 10.1016/j.ceb.2020.10.004 Peng Liu 1 , Martin Würtz 1 , Erik Zupa 1 , Stefan Pfeffer 1 , Elmar Schiebel 1
|
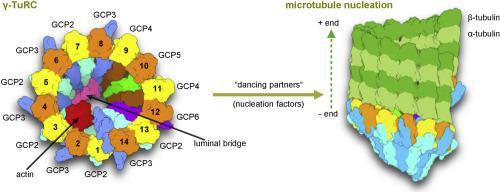
Microtubules are essential cytoskeletal elements assembled from αβ-tubulin dimers. In high eukaryotes, microtubule nucleation, the de novo assembly of a microtubule from its minus end, is initiated by the γ-tubulin ring complex (γ-TuRC). Despite many years of research, the structural and mechanistic principles of the microtubule nucleation machinery remained poorly understood. Only recently, cryoelectron microscopy studies uncovered the molecular organization and potential activation mechanisms of γ-TuRC. In vitro assays further deciphered the spatial and temporal cooperation between γ-TuRC and additional factors, for example, the augmin complex, the phase separation protein TPX2, and the microtubule polymerase XMAP215. These breakthroughs deepen our understanding of microtubule nucleation mechanisms and will link the assembly of individual microtubules to the organization of cellular microtubule networks.
中文翻译:

微管成核:γ-微管蛋白环复合物与相关蛋白之间的华尔兹
微管是由αβ-微管蛋白二聚体组装而成的必需细胞骨架元件。在高等真核生物中,微管成核,即从负端开始的微管从头组装,是由γ-微管蛋白环复合物(γ-TuRC)引发的。尽管进行了多年的研究,但对微管成核机制的结构和机理原理仍然知之甚少。直到最近,冷冻电子显微镜研究才发现γ-TuRC的分子组织和潜在的激活机制。体外分析进一步破译了γ-TuRC与其他因素(例如,augmin复合物,相分离蛋白TPX2和微管聚合酶XMAP215)之间的时空协作。这些突破加深了我们对微管成核机制的理解,并将单个微管的组装与细胞微管网络的组织联系起来。







































 京公网安备 11010802027423号
京公网安备 11010802027423号