Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-11-12 , DOI: 10.1016/j.bmcl.2020.127681 Jun-Wei Xu 1 , Yang-Li Qi 1 , Jian-Wei Wu 1 , Rui-Xiang Yuan 1 , Xiao-Wen Chen 1 , Jian-Qi Li 1
|
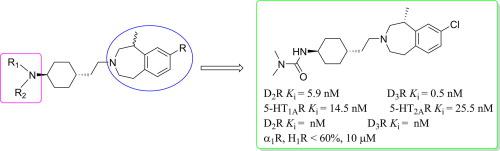
In this study, a series of trans-4-(2-(1,2,4,5-tetrahydro-3H-benzo[d]azepin-3-yl)ethyl)cyclohexan-1-amine derivatives as potential antipsychotics were synthesized and biologically evaluated to discover potential antipsychotics with good drug target selectivity. The preliminary structure-activity relationship was discussed, and optimal compound 12a showed both nanomolar affinity for D2/D3/5-HT1A/5-HT2A receptors and weak α1 and H1 receptor binding affinity. In addition, 12a was metabolically stable in vitro, displayed micromolar affinity for the hERG channel, and exhibited antipsychotic efficacy in the animal model of locomotor-stimulating effects of phencyclidine.
中文翻译:

靶向多巴胺/ 5-羟色胺的新型抗精神病药反式-4-(2-(1,2,4,5-四氢-3 H-苯并[ d ]氮杂-3-基)乙基)环己-1-胺衍生物的合成及生物学评价受体亚型
在这项研究中,作为潜在的抗精神病药的一系列反式-4-(2-(1,2,4,5-四氢-3 H-苯并[ d ]氮杂-3-基)乙基)环己-1-胺衍生物是合成并进行生物学评估以发现具有良好药物靶点选择性的潜在抗精神病药。讨论了初步结构-活性关系,和最佳化合物12A既表现出纳摩尔亲和力为d 2 / d 3 /5-HT 1A / 5-HT 2A受体和弱α 1和H 1受体的结合亲和力。此外,12a在体外代谢稳定,对hERG通道表现出微摩尔亲和力,并在苯环利定的运动刺激作用动物模型中表现出抗精神病功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号