Advanced Powder Technology ( IF 4.2 ) Pub Date : 2020-11-12 , DOI: 10.1016/j.apt.2020.10.001 S. Mishra , S.S. Sahoo , A.K. Debnath , K.P. Muthe , N. Das , P. Parhi
|
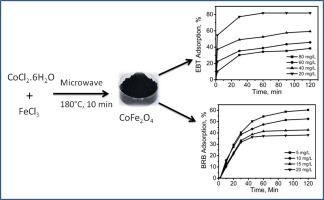
Magnetic nanoferrites (MFe2O4, M = Co, Ni) were successfully synthesised through microwave-hydrothermal route, characterised and used for adsorption of Eriochrome Black T (EBT) and Bromophenol Blue (BRB) dyes from their aqueous solution. The powder XRD patterns confirmed the formation of cubic spinel structure for both the ferrites. Under identical conditions, the adsorption efficiency of CoFe2O4 was found relatively higher than the corresponding NiFe2O4. Further characterisations revealed that CoFe2O4 sample was nearly spherical in size (8–9 nm) with narrow size distribution. The sample showed superparamagnetic behaviour with saturation magnetization (Ms) value (66.4 emu/g). BET surface area calculated for the synthesized cobalt ferrite as 70.9 m2/g. Batch adsorption experiments as a function of initial dye concentration, pH, contact time and adsorbent dose showed the adsorption of dyes depends on pH. Equilibrium adsorption data were well explained by both Langmuir and Freundlich isotherm models. The maximum monolayer adsorption capacities (Qo) were found to be 82.6 and 25.6 mg/g for EBT and BRB dyes, respectively. Kinetics of the adsorption was best described by pseudo-second-order model. Various thermodynamic parameters such as ΔG, ΔH and ΔS derived from adsorption data over the temperature range 20–50 °C, accounted for a favourable, spontaneous, endothermic physisorption process. The materials showed potential for repeated use without significant decrease in adsorption capacity after proper regeneration.
中文翻译:

微波水热法合成钴铁氧体纳米粒子及其对有机染料的吸附效率:等温线,热力学和动力学研究
通过微波-水热法成功合成了磁性纳米铁氧体(MFe 2 O 4,M = Co,Ni),表征并用于从水溶液中吸附铬铁黑T(EBT)和溴酚蓝(BRB)染料。粉末XRD图谱证实了两种铁素体均形成立方尖晶石结构。在相同条件下,发现CoFe 2 O 4的吸附效率相对高于相应的NiFe 2 O 4。进一步的特征表明CoFe 2 O 4样品尺寸接近球形(8-9 nm),尺寸分布窄。样品表现出超顺磁行为,饱和磁化强度(M s)值为(66.4 emu / g)。计算出的合成钴铁氧体的BET表面积为70.9m 2 / g。分批吸附实验作为初始染料浓度,pH,接触时间和吸附剂剂量的函数,表明染料的吸附取决于pH。Langmuir和Freundlich等温线模型很好地解释了平衡吸附数据。最大单层吸附量(Q o)对于EBT和BRB染料,分别为82.6和25.6 mg / g。吸附动力学最好用拟二阶模型描述。从20–50°C的温度范围内的吸附数据得出的各种热力学参数(例如ΔG,ΔH和ΔS)是有利的,自发的,吸热的物理吸附过程。在适当再生后,该材料显示出重复使用的潜力,而吸附能力没有明显降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号