Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-11-11 , DOI: 10.1016/j.jmb.2020.11.005 Richard M. Meade , Kimberley J. Morris , Kathryn J.C. Watt , Robert J. Williams , Jody M. Mason
|
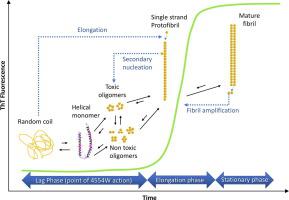
Aggregation of α-Synuclein (αS) is widely regarded as a key factor in neuronal cell death, leading to a wide range of synucleinopathies, including Parkinson’s Disease. Development of therapeutics has therefore focused on inhibiting aggregation of αS into toxic forms. One such inhibitor, based on the preNAC region αS45-54 (4554W), was identified using an intracellular peptide library screen, and subsequently shown to both inhibit formation of αS aggregates while simultaneously lowering toxicity. Subsequent efforts have sought to determine the mode of 4554W action. In particular, and consistent with the fact that both target and peptide are co-produced during library screening, we find that the peptide inhibits primary nucleation of αS, but does not modulate downstream elongation or secondary nucleation events. These findings hold significant promise towards mechanistic understanding and development of molecules that can module the first steps in αS aggregation towards novel treatments for Parkinson’s disease and related synucleinopathies.
中文翻译:

该库衍生的4554W肽抑制α-突触核蛋白的初级成核
α-突触核蛋白(αS)的聚集被广泛认为是神经元细胞死亡的关键因素,导致包括帕金森氏病在内的多种突触核病。因此,治疗剂的开发集中于抑制αS聚集成毒性形式。一种这样的酶抑制剂,基于所述preNAC区域αS 45-54(4554W),是使用细胞内肽库筛选方法鉴定的,随后显示出既抑制αS聚集体形成,又降低了毒性。随后的努力试图确定4554W的工作模式。特别是,与在库筛选过程中共同产生靶标和肽这一事实相一致,我们发现该肽抑制αS的初级成核,但不调节下游的次级成核或延伸事件。这些发现为机械理解和发展分子提供了重大希望,这些分子可以将αS聚合的第一步整合为帕金森氏病和相关突触核蛋白病的新疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号