当前位置:
X-MOL 学术
›
Comp. Mater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-Principles Study of the Effect of Doped Metal Atoms Pd, Mg and Ti on Tritium Release from Li2O (111) Surface
Computational Materials Science ( IF 3.1 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.commatsci.2020.110151 Jun Zhu , Yiyu Fang , Huagang Xiao , Xiaojun Chen , Tao Gao , Chengjian Xiao
Computational Materials Science ( IF 3.1 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.commatsci.2020.110151 Jun Zhu , Yiyu Fang , Huagang Xiao , Xiaojun Chen , Tao Gao , Chengjian Xiao
|
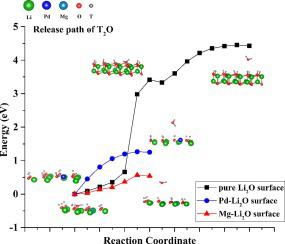
Abstract In this study, the effect of doped metal atoms Pd, Mg and Ti on the release of tritium on the Li2O (111) surface is studied by using density functional theory. On the clean Li2O (111) surface, the formation energies of T2O and T2 are 3.41 eV and 6.30 eV, respectively. About the Li2O (111) surface doped with Pd, Mg and Ti, the T2O molecule are formed spontaneously, and the desorption energies are 1.26, 0.58 and 0.18 eV, respectively. The formation energy of T2 molecule is 2.83 eV, on the surface of Ti-doped Li2O (111). By analyzing Bader charge, charge density difference (CDD), local density of state (LDOS) and the formation energy of oxygen vacancy, we found that dopants can affect the interaction of surrounding atoms through charge transfer and reduce the O vacancy formation energy, and then generate the desorption sites with low activation energy, thereby promoting the formation and desorption of T2O or T2. In a word, doping Pd, Mg and Ti on the Li2O (111) surface can catalyze the release of tritium.
中文翻译:

掺杂金属原子 Pd、Mg 和 Ti 对 Li2O (111) 表面氚释放影响的第一性原理研究
摘要 本研究利用密度泛函理论研究了掺杂金属原子Pd、Mg和Ti对Li2O(111)表面氚释放的影响。在干净的 Li2O (111) 表面,T2O 和 T2 的形成能分别为 3.41 eV 和 6.30 eV。在掺杂有 Pd、Mg 和 Ti 的 Li2O(111)表面,T2O 分子自发形成,解吸能分别为 1.26、0.58 和 0.18 eV。T2 分子的形成能是 2.83 eV,在 Ti 掺杂的 Li2O (111) 表面。通过分析Bader电荷、电荷密度差(CDD)、局部态密度(LDOS)和氧空位形成能,我们发现掺杂剂可以通过电荷转移影响周围原子的相互作用,降低O空位形成能,然后产生低活化能的解吸位点,从而促进T2O或T2的形成和解吸。总之,在Li2O(111)表面掺杂Pd、Mg和Ti可以催化氚的释放。
更新日期:2020-11-01
中文翻译:

掺杂金属原子 Pd、Mg 和 Ti 对 Li2O (111) 表面氚释放影响的第一性原理研究
摘要 本研究利用密度泛函理论研究了掺杂金属原子Pd、Mg和Ti对Li2O(111)表面氚释放的影响。在干净的 Li2O (111) 表面,T2O 和 T2 的形成能分别为 3.41 eV 和 6.30 eV。在掺杂有 Pd、Mg 和 Ti 的 Li2O(111)表面,T2O 分子自发形成,解吸能分别为 1.26、0.58 和 0.18 eV。T2 分子的形成能是 2.83 eV,在 Ti 掺杂的 Li2O (111) 表面。通过分析Bader电荷、电荷密度差(CDD)、局部态密度(LDOS)和氧空位形成能,我们发现掺杂剂可以通过电荷转移影响周围原子的相互作用,降低O空位形成能,然后产生低活化能的解吸位点,从而促进T2O或T2的形成和解吸。总之,在Li2O(111)表面掺杂Pd、Mg和Ti可以催化氚的释放。











































 京公网安备 11010802027423号
京公网安备 11010802027423号