Chemical Physics ( IF 2.0 ) Pub Date : 2020-11-11 , DOI: 10.1016/j.chemphys.2020.111047 Xueli Cheng
|
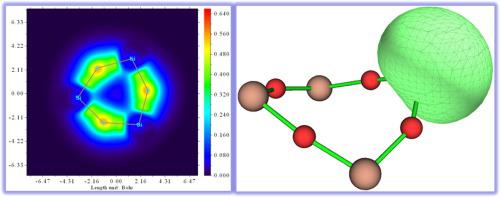
Full optimization on the ring structures of (SiO)n0, ±(n = 2–4) clusters at M06-2X-D3/def2-TZVP level demonstrates that (SiO)20, ± and (SiO)30, ± adopt planar configurations, and beginning with (SiO)40, ± the planarity of the rings is destroyed. The LOL-π analyses show that, Si atoms prevent the lone-pair electrons on O delocalizing to the whole rings. Localized molecular orbitals (LMOs) confirm that, after the two 3p electrons of a Si atom covalently bonded to two O atoms, the 2p lone-pair electrons of O atoms can diffuse to some extent to adjacent atoms to form π orbitals, which is also consolidated by orbital composition analyses. The Mayer bond order analyses and multi-center bond order analyses are also discussed in the present work.
中文翻译:

对中性和离子性(SiO)n 0,±(n = 2-4)的环结构和芳香性的理论见解
在M06-2X-D3 / def2-TZVP水平上对(SiO)n 0,±(n = 2-4)团簇的环结构的完全优化表明,(SiO)2 0,±和(SiO)3 0,±采用平面配置,并以(SiO)4 0,±开头环的平面度被破坏。LOL-π分析表明,Si原子阻止O上的孤对电子离域到整个环。局部分子轨道(LMOs)证实,在一个Si原子的两个3p电子共价键合到两个O原子之后,O原子的2p孤对电子可以在一定程度上扩散到相邻原子以形成π轨道,这也是通过轨道组成分析进行合并。Mayer债券订单分析和多中心债券订单分析也将在本工作中进行讨论。











































 京公网安备 11010802027423号
京公网安备 11010802027423号