当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 5-substituted tetrazoles via DNA-conjugated nitrile
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-09 , DOI: 10.1039/d0ob02021d Huang-Chi Du 1 , Martin M Matzuk 1 , Ying-Chu Chen 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-09 , DOI: 10.1039/d0ob02021d Huang-Chi Du 1 , Martin M Matzuk 1 , Ying-Chu Chen 1
Affiliation
|
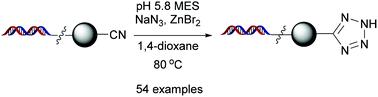
A zinc bromide-catalyzed synthesis of 5-substituted tetrazoles via DNA-conjugated nitriles using sodium azide has been developed. The protocol offered moderate to excellent yields of tetrazoles with a broad range of substrates, including a variety of functionalized aromatic, heterocyclic, and aliphatic nitriles. In addition, the electronic effect within the substrate scope was evaluated. DNA fidelity was assessed by ligation efficiency and amplifiability analysis. The ability to generate tetrazoles expands the diversity of heterocycles in the preparation of DNA-encoded chemical libraries.
中文翻译:

DNA共轭腈合成5-取代四唑
已经开发了使用叠氮化钠通过DNA 共轭腈合成 5-取代四唑的溴化锌。该协议提供了中等至优异的四唑产量,具有广泛的底物,包括各种功能化的芳族、杂环和脂肪族腈。此外,评价了基板范围内的电子效应。DNA保真度通过连接效率和扩增性分析进行评估。在制备 DNA 编码的化学文库时,产生四唑的能力扩大了杂环的多样性。
更新日期:2020-11-12
中文翻译:

DNA共轭腈合成5-取代四唑
已经开发了使用叠氮化钠通过DNA 共轭腈合成 5-取代四唑的溴化锌。该协议提供了中等至优异的四唑产量,具有广泛的底物,包括各种功能化的芳族、杂环和脂肪族腈。此外,评价了基板范围内的电子效应。DNA保真度通过连接效率和扩增性分析进行评估。在制备 DNA 编码的化学文库时,产生四唑的能力扩大了杂环的多样性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号