当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Supramolecular protein polymers using mini-ferritin Dps as the building block
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0ob01702g M Raquel Pacheco 1 , João P Jacinto 1 , Daniela Penas 1 , Tomás Calmeiro 2 , Ana V Almeida 1 , Miriam Colaço 1 , Elvira Fortunato 2 , Nykola C Jones 3 , Søren V Hoffmann 3 , M Manuela A Pereira 4 , Pedro Tavares 1 , Alice S Pereira 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0ob01702g M Raquel Pacheco 1 , João P Jacinto 1 , Daniela Penas 1 , Tomás Calmeiro 2 , Ana V Almeida 1 , Miriam Colaço 1 , Elvira Fortunato 2 , Nykola C Jones 3 , Søren V Hoffmann 3 , M Manuela A Pereira 4 , Pedro Tavares 1 , Alice S Pereira 1
Affiliation
|
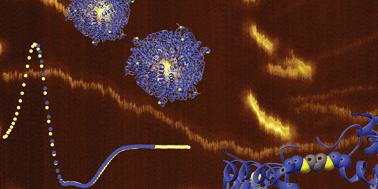
A missense mutant of a Dps protein (DNA-binding protein from starved cells) from Marinobacter hydrocarbonoclasticus was used as a building block to develop a new supramolecular assembly complex which enhances the iron uptake, a physiological function of this mini-ferritin. The missense mutation was conducted in an exposed and flexible region of the N-terminal, wherein a threonine residue in position 10 was replaced by a cysteine residue (DpsT10C). This step enabled a click chemistry approach to the variant DpsT10C, where a thiol–ene coupling occurs. Two methods and two types of linker were used resulting in two different mini-ferritin supramolecular polymers, which have maintained secondary structure and native iron uptake physiological function. Electrophoretic assays and mass spectrometry were utilized to confirm that both functionalization and coupling reactions occured as predicted. The secondary structure has been investigated by circular dichroism and synchrotron radiation circular dichroism. Size and morphology were obtained by dynamic light scattering, size exclusion chromatography and atomic force microscopy, respectively. The iron uptake of the synthesized protein polymers was confirmed by UV-Vis spectroscopy loading assays.
中文翻译:

以微型铁蛋白 Dps 为基础的超分子蛋白质聚合物
来自海洋碎烃杆菌的 Dps 蛋白(来自饥饿细胞的 DNA 结合蛋白)的错义突变体被用作构建块以开发一种新的超分子组装复合物,该复合物可增强铁的吸收,这是这种微型铁蛋白的一种生理功能。错义突变在 N 末端的暴露和柔性区域中进行,其中第 10 位的苏氨酸残基被半胱氨酸残基 (DpsT10C) 取代。此步骤启用了对变体 DpsT10C 的点击化学方法,其中发生硫醇-烯偶联。使用了两种方法和两种类型的接头,产生了两种不同的微型铁蛋白超分子聚合物,它们保持了二级结构和天然铁摄取生理功能。电泳测定法和质谱法用于确认功能化和偶联反应都如预测的那样发生。通过圆二色性和同步辐射圆二色性研究了二级结构。尺寸和形态分别通过动态光散射、尺寸排阻色谱和原子力显微镜获得。合成的蛋白质聚合物的铁吸收通过紫外-可见光谱加载测定得到证实。
更新日期:2020-11-12
中文翻译:

以微型铁蛋白 Dps 为基础的超分子蛋白质聚合物
来自海洋碎烃杆菌的 Dps 蛋白(来自饥饿细胞的 DNA 结合蛋白)的错义突变体被用作构建块以开发一种新的超分子组装复合物,该复合物可增强铁的吸收,这是这种微型铁蛋白的一种生理功能。错义突变在 N 末端的暴露和柔性区域中进行,其中第 10 位的苏氨酸残基被半胱氨酸残基 (DpsT10C) 取代。此步骤启用了对变体 DpsT10C 的点击化学方法,其中发生硫醇-烯偶联。使用了两种方法和两种类型的接头,产生了两种不同的微型铁蛋白超分子聚合物,它们保持了二级结构和天然铁摄取生理功能。电泳测定法和质谱法用于确认功能化和偶联反应都如预测的那样发生。通过圆二色性和同步辐射圆二色性研究了二级结构。尺寸和形态分别通过动态光散射、尺寸排阻色谱和原子力显微镜获得。合成的蛋白质聚合物的铁吸收通过紫外-可见光谱加载测定得到证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号