当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrogen and oxygen tailoring of a solid carbon active site for two-electron selectivity electrocatalysis
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-10-29 , DOI: 10.1039/d0qi01089h Hongyang Shao 1, 2, 3, 4, 5 , Quan Zhuang 1, 2, 3, 4, 5 , Hongda Gao 5, 6, 7, 8 , Yin Wang 1, 2, 3, 4, 5 , Lei Ji 1, 2, 3, 4, 5 , Xia Wang 1, 2, 3, 4, 5 , Tingting Zhang 1, 2, 3, 4, 5 , Limei Duan 1, 2, 3, 4, 5 , Jie Bai 5, 6, 7, 8 , Zhiqiang Niu 9, 10, 11, 12, 13 , Jinghai Liu 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-10-29 , DOI: 10.1039/d0qi01089h Hongyang Shao 1, 2, 3, 4, 5 , Quan Zhuang 1, 2, 3, 4, 5 , Hongda Gao 5, 6, 7, 8 , Yin Wang 1, 2, 3, 4, 5 , Lei Ji 1, 2, 3, 4, 5 , Xia Wang 1, 2, 3, 4, 5 , Tingting Zhang 1, 2, 3, 4, 5 , Limei Duan 1, 2, 3, 4, 5 , Jie Bai 5, 6, 7, 8 , Zhiqiang Niu 9, 10, 11, 12, 13 , Jinghai Liu 1, 2, 3, 4, 5
Affiliation
|
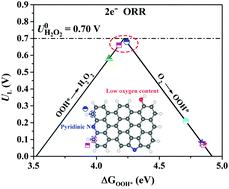
The synthesis of hydrogen peroxide (H2O2) via two-electron (2e−) oxygen reduction reaction (ORR) electrocatalysis has garnered extensive attention as an appealing green and safe production technology. However, the key progress is still strongly dependent on developing highly active, selective and stable electrocatalysts. Here, guided by first-principles calculations, we developed a rational design for nitrogen (N) and oxygen (O) tailoring of carbon materials by precisely controlling the pyridinic N (Pydn-N) structure and the oxygen doping. The second nearest C atoms of Pydn-N were identified to be the active site, and this is suppressed owing to its local chemical environment and increase in the oxygen dopant content. Experimentally, high-temperature hydrogenation (HTH) regulates the Pydn-N content and synchronous oxygen removal for N,O co-doped carbons (CNO-x). The increment of Pydn-N from 37.42% up to 39.73%, along with oxygen removal from 5.24 at% down to 3.80 at% after HTH, promotes the H2O2 selectivity and electron transfer number (n) to 82% and 2.36. The H2O2 production rate is stable at around 200 mmol gcat−1 h−1 and a faradaic efficiency (F.E.) of up to 80% was recorded during the initial 4 h. The long-term H2O2 production further highlights the significance of sustaining the Pydn-N structure, modulating the solid carbon active site.
中文翻译:

固碳活性位的氮和氧调节用于两电子选择性电催化
过氧化氢(H的合成2 ö 2)通过两电子(2E -氧还原反应(ORR)电催化作为一种吸引人的绿色安全生产技术已引起广泛关注。然而,关键的进展仍然强烈地依赖于开发高活性,选择性和稳定的电催化剂。在此,以第一性原理计算为指导,我们通过精确控制吡啶氮(Pydn-N)结构和氧掺杂,开发了碳材料氮(N)和氧(O)定制的合理设计。Pydn-N的倒数第二个C原子被确定为活性位点,由于其局部化学环境和氧掺杂剂含量的增加,这一点被抑制了。实验上,高温氢化(HTH)调节Pydn-N含量,并同步去除N,O共掺杂碳(CNO-x)的氧气。Pydn-N的增加从37.42%上升到39。2 O 2的选择性和电子转移数( n)分别为82%和2.36。H 2 O 2的产生速率稳定在200 mmol g cat -1 h -1左右,并且在最初的4 h内记录的法拉第效率(FE)高达80%。长期生产H 2 O 2进一步突出了维持Pydn-N结构,调节固体碳活性位点的重要性。
更新日期:2020-11-12
中文翻译:

固碳活性位的氮和氧调节用于两电子选择性电催化
过氧化氢(H的合成2 ö 2)通过两电子(2E -氧还原反应(ORR)电催化作为一种吸引人的绿色安全生产技术已引起广泛关注。然而,关键的进展仍然强烈地依赖于开发高活性,选择性和稳定的电催化剂。在此,以第一性原理计算为指导,我们通过精确控制吡啶氮(Pydn-N)结构和氧掺杂,开发了碳材料氮(N)和氧(O)定制的合理设计。Pydn-N的倒数第二个C原子被确定为活性位点,由于其局部化学环境和氧掺杂剂含量的增加,这一点被抑制了。实验上,高温氢化(HTH)调节Pydn-N含量,并同步去除N,O共掺杂碳(CNO-x)的氧气。Pydn-N的增加从37.42%上升到39。2 O 2的选择性和电子转移数( n)分别为82%和2.36。H 2 O 2的产生速率稳定在200 mmol g cat -1 h -1左右,并且在最初的4 h内记录的法拉第效率(FE)高达80%。长期生产H 2 O 2进一步突出了维持Pydn-N结构,调节固体碳活性位点的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号