当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and self-assembly of aminyl and alkynyl substituted sophorolipids
Green Chemistry ( IF 9.8 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0gc03053h Abdoul Aziz Ba 1, 2, 3, 4, 5 , Jonas Everaert 6, 7, 8, 9, 10 , Alexandre Poirier 1, 2, 3, 4, 5 , Patrick Le Griel 1, 2, 3, 4, 5 , Wim Soetaert 8, 9, 10, 11, 12 , Sophie L. K. W. Roelants 8, 9, 10, 11, 12 , Daniel Hermida-Merino 13, 14, 15 , Christian V. Stevens 6, 7, 8, 9, 10 , Niki Baccile 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0gc03053h Abdoul Aziz Ba 1, 2, 3, 4, 5 , Jonas Everaert 6, 7, 8, 9, 10 , Alexandre Poirier 1, 2, 3, 4, 5 , Patrick Le Griel 1, 2, 3, 4, 5 , Wim Soetaert 8, 9, 10, 11, 12 , Sophie L. K. W. Roelants 8, 9, 10, 11, 12 , Daniel Hermida-Merino 13, 14, 15 , Christian V. Stevens 6, 7, 8, 9, 10 , Niki Baccile 1, 2, 3, 4, 5
Affiliation
|
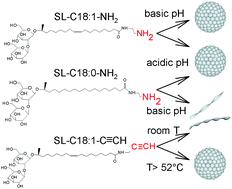
Sophorolipids are one of the most important microbial biosurfactants, because of their large-scale production and applications developed so far in the fields of detergency, microbiology, cosmetics or environmental science. However, the structural variety of native sophorolipids is limited/restricted, a limiting fact for the development of new properties and their potential applications. In their open acidic form, C18:1 sophorolipids (SL) are classically composed of a sophorose headgroup and a carboxylic acid (COOH) end-group. The carboxyl group gives them unique pH-responsive properties, but it is a poorly reactive group and its charge can only be negative. To develop a new generation of pH-responsive, positively charged, SL and to improve their reactivity for further functionalization, we develop here SL with an amine (–NH2) or terminal alkyne (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) end-group analogues. The amine group generates positively charged SL and is more reactive than carboxylic acids, e.g. towards aldehydes; the alkyne group provides access to copper-based click chemistry. In this work, we synthesized (C18:1) and (C18:0) –NH2 and (C18:1) –C
CH) end-group analogues. The amine group generates positively charged SL and is more reactive than carboxylic acids, e.g. towards aldehydes; the alkyne group provides access to copper-based click chemistry. In this work, we synthesized (C18:1) and (C18:0) –NH2 and (C18:1) –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH sophorolipid derivatives and we study their self-assembly properties in response to pH and/or temperature changes by means of static and dynamic light scattering, small angle (X-ray, neutron) scattering and cryogenic electron microscopy. Monounsaturated aminyl SL-C18:1-NH2 sophorolipids form a micellar phase in their neutral form at high pH and a mixed micellar-bilayer phase in their positively charged form at low pH. Saturated aminyl SL-C18:0-NH2 sophorolipids form a micellar phase in their charged form at low pH and a twisted ribbon phase in their neutral form at high pH and monounsaturated alkynyl SL-C18:1-C
CH sophorolipid derivatives and we study their self-assembly properties in response to pH and/or temperature changes by means of static and dynamic light scattering, small angle (X-ray, neutron) scattering and cryogenic electron microscopy. Monounsaturated aminyl SL-C18:1-NH2 sophorolipids form a micellar phase in their neutral form at high pH and a mixed micellar-bilayer phase in their positively charged form at low pH. Saturated aminyl SL-C18:0-NH2 sophorolipids form a micellar phase in their charged form at low pH and a twisted ribbon phase in their neutral form at high pH and monounsaturated alkynyl SL-C18:1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH sophorolipids form a main micellar phase at T > 51.8 °C and a twisted ribbon phase at T < 51.8 °C.
CH sophorolipids form a main micellar phase at T > 51.8 °C and a twisted ribbon phase at T < 51.8 °C.
中文翻译:

氨基和炔基取代的槐糖脂的合成与自组装
槐糖脂是最重要的微生物生物表面活性剂之一,因为迄今为止它们在洗涤剂,微生物学,化妆品或环境科学领域中已得到大规模生产和应用。然而,天然槐糖脂的结构多样性受到限制/限制,这是开发新特性及其潜在应用的一个限制事实。C18:1槐糖脂(SL)以开放的酸性形式经典地由槐糖糖头基和羧酸(COOH)端基组成。羧基赋予它们独特的pH响应特性,但它是反应性较差的基团,其电荷只能为负。为了开发新一代的pH响应,带正电荷的SL并提高其反应性以进一步官能化,我们在这里开发了一种带有胺(–NH2)或末端炔烃(–C![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) CH)端基类似物。胺基生成带正电荷的SL,并且比羧酸更具反应性,例如对醛而言;炔烃基团提供了使用铜基点击化学的途径。在这项工作中,我们合成了(C18:1)和(C18:0)–NH 2和(C18:1)–C
CH)端基类似物。胺基生成带正电荷的SL,并且比羧酸更具反应性,例如对醛而言;炔烃基团提供了使用铜基点击化学的途径。在这项工作中,我们合成了(C18:1)和(C18:0)–NH 2和(C18:1)–C![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) CH槐糖脂衍生物,并通过以下方法研究了它们对pH和/或温度变化的响应的自组装特性静态和动态光散射,小角度(X射线,中子)散射和低温电子显微镜的研究。单不饱和胺基SL-C18:1-NH 2槐糖脂在高pH下以中性形式形成胶束相,在低pH下以正电荷形式形成混合胶束双层相。在低pH值下,饱和的氨基SL-C18:0-NH 2槐糖脂以带电形式形成胶束相,在高pH值下,它们以中性形式形成扭曲的带状相,单不饱和炔基SL-C18:1-C
CH槐糖脂衍生物,并通过以下方法研究了它们对pH和/或温度变化的响应的自组装特性静态和动态光散射,小角度(X射线,中子)散射和低温电子显微镜的研究。单不饱和胺基SL-C18:1-NH 2槐糖脂在高pH下以中性形式形成胶束相,在低pH下以正电荷形式形成混合胶束双层相。在低pH值下,饱和的氨基SL-C18:0-NH 2槐糖脂以带电形式形成胶束相,在高pH值下,它们以中性形式形成扭曲的带状相,单不饱和炔基SL-C18:1-C ![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) CH槐糖脂形成主要的胶束。相在Ť > 51.8℃,并在扭绞带相Ť 51.8°C <。
CH槐糖脂形成主要的胶束。相在Ť > 51.8℃,并在扭绞带相Ť 51.8°C <。
更新日期:2020-11-12
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) end-group analogues. The amine group generates positively charged SL and is more reactive than carboxylic acids, e.g. towards aldehydes; the alkyne group provides access to copper-based click chemistry. In this work, we synthesized (C18:1) and (C18:0) –NH2 and (C18:1) –C
CH) end-group analogues. The amine group generates positively charged SL and is more reactive than carboxylic acids, e.g. towards aldehydes; the alkyne group provides access to copper-based click chemistry. In this work, we synthesized (C18:1) and (C18:0) –NH2 and (C18:1) –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH sophorolipid derivatives and we study their self-assembly properties in response to pH and/or temperature changes by means of static and dynamic light scattering, small angle (X-ray, neutron) scattering and cryogenic electron microscopy. Monounsaturated aminyl SL-C18:1-NH2 sophorolipids form a micellar phase in their neutral form at high pH and a mixed micellar-bilayer phase in their positively charged form at low pH. Saturated aminyl SL-C18:0-NH2 sophorolipids form a micellar phase in their charged form at low pH and a twisted ribbon phase in their neutral form at high pH and monounsaturated alkynyl SL-C18:1-C
CH sophorolipid derivatives and we study their self-assembly properties in response to pH and/or temperature changes by means of static and dynamic light scattering, small angle (X-ray, neutron) scattering and cryogenic electron microscopy. Monounsaturated aminyl SL-C18:1-NH2 sophorolipids form a micellar phase in their neutral form at high pH and a mixed micellar-bilayer phase in their positively charged form at low pH. Saturated aminyl SL-C18:0-NH2 sophorolipids form a micellar phase in their charged form at low pH and a twisted ribbon phase in their neutral form at high pH and monounsaturated alkynyl SL-C18:1-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH sophorolipids form a main micellar phase at T > 51.8 °C and a twisted ribbon phase at T < 51.8 °C.
CH sophorolipids form a main micellar phase at T > 51.8 °C and a twisted ribbon phase at T < 51.8 °C.
中文翻译:

氨基和炔基取代的槐糖脂的合成与自组装
槐糖脂是最重要的微生物生物表面活性剂之一,因为迄今为止它们在洗涤剂,微生物学,化妆品或环境科学领域中已得到大规模生产和应用。然而,天然槐糖脂的结构多样性受到限制/限制,这是开发新特性及其潜在应用的一个限制事实。C18:1槐糖脂(SL)以开放的酸性形式经典地由槐糖糖头基和羧酸(COOH)端基组成。羧基赋予它们独特的pH响应特性,但它是反应性较差的基团,其电荷只能为负。为了开发新一代的pH响应,带正电荷的SL并提高其反应性以进一步官能化,我们在这里开发了一种带有胺(–NH2)或末端炔烃(–C
![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) CH)端基类似物。胺基生成带正电荷的SL,并且比羧酸更具反应性,例如对醛而言;炔烃基团提供了使用铜基点击化学的途径。在这项工作中,我们合成了(C18:1)和(C18:0)–NH 2和(C18:1)–C
CH)端基类似物。胺基生成带正电荷的SL,并且比羧酸更具反应性,例如对醛而言;炔烃基团提供了使用铜基点击化学的途径。在这项工作中,我们合成了(C18:1)和(C18:0)–NH 2和(C18:1)–C![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) CH槐糖脂衍生物,并通过以下方法研究了它们对pH和/或温度变化的响应的自组装特性静态和动态光散射,小角度(X射线,中子)散射和低温电子显微镜的研究。单不饱和胺基SL-C18:1-NH 2槐糖脂在高pH下以中性形式形成胶束相,在低pH下以正电荷形式形成混合胶束双层相。在低pH值下,饱和的氨基SL-C18:0-NH 2槐糖脂以带电形式形成胶束相,在高pH值下,它们以中性形式形成扭曲的带状相,单不饱和炔基SL-C18:1-C
CH槐糖脂衍生物,并通过以下方法研究了它们对pH和/或温度变化的响应的自组装特性静态和动态光散射,小角度(X射线,中子)散射和低温电子显微镜的研究。单不饱和胺基SL-C18:1-NH 2槐糖脂在高pH下以中性形式形成胶束相,在低pH下以正电荷形式形成混合胶束双层相。在低pH值下,饱和的氨基SL-C18:0-NH 2槐糖脂以带电形式形成胶束相,在高pH值下,它们以中性形式形成扭曲的带状相,单不饱和炔基SL-C18:1-C ![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) CH槐糖脂形成主要的胶束。相在Ť > 51.8℃,并在扭绞带相Ť 51.8°C <。
CH槐糖脂形成主要的胶束。相在Ť > 51.8℃,并在扭绞带相Ť 51.8°C <。



























 京公网安备 11010802027423号
京公网安备 11010802027423号