当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ observation of pH change during water splitting in neutral pH conditions: impact of natural convection driven by buoyancy effects
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0ee01760d Keisuke Obata 1, 2, 3, 4 , Roel van de Krol 1, 2, 3, 4, 5 , Michael Schwarze 4, 5, 5, 6, 7 , Reinhard Schomäcker 4, 5, 6, 7 , Fatwa F. Abdi 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0ee01760d Keisuke Obata 1, 2, 3, 4 , Roel van de Krol 1, 2, 3, 4, 5 , Michael Schwarze 4, 5, 5, 6, 7 , Reinhard Schomäcker 4, 5, 6, 7 , Fatwa F. Abdi 1, 2, 3, 4
Affiliation
|
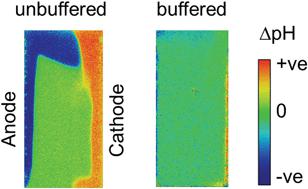
Photoelectrochemical water splitting in near-neutral pH conditions offers a safe and sustainable way to produce solar fuels, but operation at near-neutral pH is challenging because of the added concentration overpotentials due to mass-transport limitations of protons and hydroxide ions. Understanding the extent of this limitation is essential in designing a highly efficient solar fuel conversion device. In the present study, the local pH between the anode and cathode in a water splitting cell is monitored in situ using fluorescence pH sensor foils. By this direct visualization, we confirm that supporting buffer ions effectively suppress local pH changes, and we show that electrochemical reactions induce natural electrolyte convection in a non-stirred cell. The observed electrolyte convection at low current densities (<2 mA cm−2) originates from buoyancy effects due to the change in the local electrolyte density by ion depletion and accumulation. A multiphysics simulation that includes the buoyancy effect reveals that natural convection driven by electrochemical reactions stabilizes the local pH, which is consistent with our experimental observations. In contrast, the model without the buoyancy effect predicts significant shifts of the local pH away from the pKa of the buffer, even at low current densities. This experimentally validated model reveals that natural convection induced by electrochemical reactions significantly affects the overall mass-transport, especially in close vicinity of the electrodes, and it should, therefore, be considered in the design and evaluation of solar fuel conversion devices.
中文翻译:

在中性pH条件下水分解过程中pH值的原位观察:浮力效应驱动自然对流的影响
在接近中性pH的条件下进行光电化学水分解提供了一种安全且可持续的生产太阳能燃料的方法,但由于质子和氢氧根离子的质量迁移限制而增加了浓度超电势,因此在接近中性pH的条件下运行具有挑战性。在设计高效的太阳能转换设备时,了解此限制的程度至关重要。在本研究中,对水分解池中阳极和阴极之间的局部pH进行原位监测使用荧光pH传感器箔。通过这种直接的可视化,我们确认支持缓冲离子有效地抑制了局部pH的变化,并且我们表明电化学反应在非搅拌的电池中诱导了自然的电解质对流。在低电流密度(<2 mA cm -2)时,观察到的电解质对流源自浮力效应,这是由于离子消耗和累积引起的局部电解质密度变化而引起的浮力效应。包含浮力效应的多物理场模拟表明,由电化学反应驱动的自然对流可稳定局部pH值,这与我们的实验观察结果一致。相反,没有浮力作用的模型预测局部pH值明显偏离p K a。即使在低电流密度的情况下也能保持良好的缓冲性能。这个经过实验验证的模型表明,电化学反应引起的自然对流会显着影响整体的传质,尤其是在电极附近,因此,在设计和评估太阳能转换装置时应考虑该对流。
更新日期:2020-11-12
中文翻译:

在中性pH条件下水分解过程中pH值的原位观察:浮力效应驱动自然对流的影响
在接近中性pH的条件下进行光电化学水分解提供了一种安全且可持续的生产太阳能燃料的方法,但由于质子和氢氧根离子的质量迁移限制而增加了浓度超电势,因此在接近中性pH的条件下运行具有挑战性。在设计高效的太阳能转换设备时,了解此限制的程度至关重要。在本研究中,对水分解池中阳极和阴极之间的局部pH进行原位监测使用荧光pH传感器箔。通过这种直接的可视化,我们确认支持缓冲离子有效地抑制了局部pH的变化,并且我们表明电化学反应在非搅拌的电池中诱导了自然的电解质对流。在低电流密度(<2 mA cm -2)时,观察到的电解质对流源自浮力效应,这是由于离子消耗和累积引起的局部电解质密度变化而引起的浮力效应。包含浮力效应的多物理场模拟表明,由电化学反应驱动的自然对流可稳定局部pH值,这与我们的实验观察结果一致。相反,没有浮力作用的模型预测局部pH值明显偏离p K a。即使在低电流密度的情况下也能保持良好的缓冲性能。这个经过实验验证的模型表明,电化学反应引起的自然对流会显着影响整体的传质,尤其是在电极附近,因此,在设计和评估太阳能转换装置时应考虑该对流。











































 京公网安备 11010802027423号
京公网安备 11010802027423号