当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Density Functional Theory Mechanistic Study on H2O2 Production Using an Organic Semiconductor Epindolidione
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.jpca.0c08496 Nitin Wadnerkar 1 , Viktor Gueskine 1 , Eric Daniel Głowacki 1, 2, 3 , Igor Zozoulenko 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.jpca.0c08496 Nitin Wadnerkar 1 , Viktor Gueskine 1 , Eric Daniel Głowacki 1, 2, 3 , Igor Zozoulenko 1
Affiliation
|
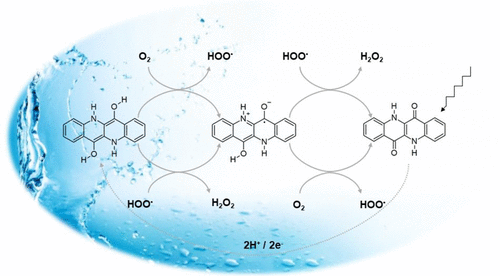
Organic semiconductors have recently emerged as promising catalytic materials for oxygen reduction to hydrogen peroxide, H2O2, a chemical of great importance in industry as well as biology. While examples of organic semiconductor-mediated photocatalytic and electrocatalytic processes for H2O2 production become more numerous and improve in performance, fundamental understanding of the reaction mechanisms at play have been explored far less. The aim of the present work is to computationally test hypotheses of how selective oxygen reduction to H2O2 generally occurs on carbonyl dyes and pigments. As an example material, we consider epindolidione (EPI), an industrial pigment with demonstrated semiconductor properties, which photocatalytic activity in oxygen reduction reaction (ORR) and thereby producing hydrogen peroxide (H2O2) in low pH environment has been recently experimentally demonstrated. In this work, the ability of the reduced form of EPI, viz. EPI-2H (which was formed after a photoinduced 2e–/2H+ process), to reduce molecular triplet oxygen to peroxide and the possible mechanism of this reaction are computationally investigated using density functional theory. In the main reaction pathway, the reduction of O2 to H2O2 reaction occurs via abstraction of one of the hydrogen atoms of EPI-2H by triplet dioxygen to produce an intermediate complex consisting of the radicals of hydrogen peroxide (HOO•) and EPI-H• at the initial stage. HOO• thus released can abstract another hydrogen atom from EPI-H• to produce H2O2 and regenerates EPI; otherwise, it can enter another pathway to abstract hydrogen from a neighboring EPI-2H to form EPI-H• and H2O2. EPI, after reduction, thus plays in ORR the role of hydrogen atom transfer (HAT) agent via its OH group, similar to anthraquinone in the industrial process, while HAT from its amino hydrogen is found unfavorable.
中文翻译:

使用有机半导体埃潘多地酮生产H 2 O 2的密度泛函理论机理研究
最近,有机半导体已成为将氧气还原为过氧化氢H 2 O 2的有前途的催化材料,而过氧化氢H 2 O 2在工业和生物学中均具有重要意义。尽管用于生产H 2 O 2的有机半导体介导的光催化和电催化方法的例子越来越多,并且性能得到了改善,但对起作用的反应机理的基本了解却很少。本工作的目的是通过计算检验关于如何将氧气选择性还原为H 2 O 2的假设通常发生在羰基染料和颜料上。作为示例材料,我们考虑了表皮二酮(EPI),这是一种具有证明的半导体特性的工业颜料,最近在实验上证明了其在低pH环境中在氧还原反应(ORR)中具有光催化活性从而产生过氧化氢(H 2 O 2)。 。在这项工作中,EPI简化形式的功能即。EPI-2H(由光诱导的2e – / 2H +过程形成)将分子三重态氧还原成过氧化物,并且使用密度泛函理论研究了该反应的可能机理。在主要反应途径中,O 2还原为H 2 O2反应是通过三重态双氧抽象化EPI-2H的一个氢原子而发生的,从而在初始阶段产生了由过氧化氢(HOO •)和EPI-H •自由基组成的中间配合物。这样释放的HOO •可以从EPI-H •提取另一个氢原子,从而产生H 2 O 2并再生EPI;否则,它可以进入另一条途径,从相邻的EPI-2H提取氢以形成EPI-H •和H 2 O 2。。还原后,EPI在ORR中通过氢原子转移(HAT)剂通过其OH基发挥作用,类似于工业过程中的蒽醌,而发现其氨基氢中的HAT则不利。
更新日期:2020-11-19
中文翻译:

使用有机半导体埃潘多地酮生产H 2 O 2的密度泛函理论机理研究
最近,有机半导体已成为将氧气还原为过氧化氢H 2 O 2的有前途的催化材料,而过氧化氢H 2 O 2在工业和生物学中均具有重要意义。尽管用于生产H 2 O 2的有机半导体介导的光催化和电催化方法的例子越来越多,并且性能得到了改善,但对起作用的反应机理的基本了解却很少。本工作的目的是通过计算检验关于如何将氧气选择性还原为H 2 O 2的假设通常发生在羰基染料和颜料上。作为示例材料,我们考虑了表皮二酮(EPI),这是一种具有证明的半导体特性的工业颜料,最近在实验上证明了其在低pH环境中在氧还原反应(ORR)中具有光催化活性从而产生过氧化氢(H 2 O 2)。 。在这项工作中,EPI简化形式的功能即。EPI-2H(由光诱导的2e – / 2H +过程形成)将分子三重态氧还原成过氧化物,并且使用密度泛函理论研究了该反应的可能机理。在主要反应途径中,O 2还原为H 2 O2反应是通过三重态双氧抽象化EPI-2H的一个氢原子而发生的,从而在初始阶段产生了由过氧化氢(HOO •)和EPI-H •自由基组成的中间配合物。这样释放的HOO •可以从EPI-H •提取另一个氢原子,从而产生H 2 O 2并再生EPI;否则,它可以进入另一条途径,从相邻的EPI-2H提取氢以形成EPI-H •和H 2 O 2。。还原后,EPI在ORR中通过氢原子转移(HAT)剂通过其OH基发挥作用,类似于工业过程中的蒽醌,而发现其氨基氢中的HAT则不利。











































 京公网安备 11010802027423号
京公网安备 11010802027423号