当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
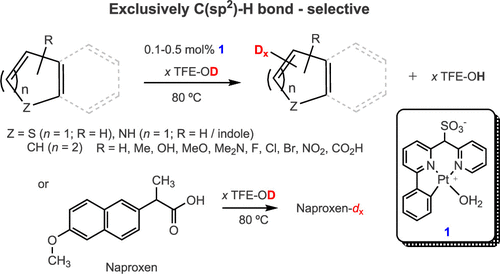
Catalytic Deuteration of C(sp2)–H Bonds of Substituted (Hetero)arenes in a Pt(II) CNN-Pincer Complex/2,2,2-Trifluoroethanol-d1 System: Effect of Substituents on the Reaction Rate and Selectivity
Organometallics ( IF 2.8 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.organomet.0c00652 Morgan Kramer 1 , David Watts 1 , Andrei N. Vedernikov 1
Organometallics ( IF 2.8 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.organomet.0c00652 Morgan Kramer 1 , David Watts 1 , Andrei N. Vedernikov 1
Affiliation
|
Thirty four (hetero)arene derivatives have been tested in catalytic H/D exchange reactions involving their C(sp2)–H bonds and 2,2,2-trifluoroethanol-d1 (TFE-d1) in the presence of the homogeneous Pt(II) complex 1 supported by a sulfonated CNN-pincer ligand at 80 °C. The 18 substrates, including one pharmaceutical (naproxen), that are stable in the presence of 1 and are active in the H/D exchange reaction have been characterized by their position-specific extent of deuteration and, in a number of cases, the reaction kinetic selectivity. For the most reactive substrates the extent of deuteration approaches the expected statistical distribution of the exchangeable H and D atoms: e.g., 67–69% for phenol after 23 h and 88% for indole β-CH bonds after 45 min. For a few substrates (N,N-dimethylaniline, indole, nitrobenzene) the H/D exchange is highly position selective. No satisfactory correlation was found between the position-specific (meta, para) H/D exchange rate constants for X-monosubstituted benzenes and Hammett σX constants. This observation was proposed to be related to the concerted nature of the CH bond activation, the rate-determining CH bond oxidative addition at a Pt(II) center. A novel scale of Hammett σMX constants was introduced to characterize the reactivity of C(sp2)–H bonds in transition-metal-mediated reactions. The experimentally determined position-specific Gibbs energies of activation of the H/D exchange in substituted benzenes (meta and para positions) as well as in thiophene (α and β positions) were matched satisfactorily using DFT calculations.
中文翻译:

Pt(II)CNN-Pincer配合物/ 2,2,2-三氟乙醇-d 1体系中取代(杂)芳烃的C(sp 2)-H键的催化氘代:取代基对反应速率和选择性的影响
已经在均相存在下,在涉及其C(sp 2)-H键和2,2,2-三氟乙醇-d 1(TFE- d 1)的催化H / D交换反应中测试了34种(杂)芳烃衍生物。Pt(II)配合物1在80°C受磺化CNN-钳夹配体支撑。18种底物,包括一种药物(萘普生),在1种存在下稳定它们在H / D交换反应中的活性已通过其特定位置的氘化程度以及在许多情况下的反应动力学选择性来表征。对于反应性最强的底物,氘的程度接近可交换H和D原子的预期统计分布:例如23小时后苯酚的67-69%和45分钟后吲哚β-CH键的88%。对于一些底物(N,N-二甲基苯胺,吲哚,硝基苯),H / D交换具有很高的位置选择性。没有令人满意的相关性被发现位置特异性的(间间,对位)H / d交换速率常数X单取代的苯和哈米特σ X常数。提出该观察结果与CH键活化的协调性质有关,CH键活化的速率决定了Pt(II)中心的CH键氧化加成。哈米特σ的一种新颖的规模中号X常数被引入来表征C(SP的反应2中的过渡金属介导的反应)-H键。使用DFT计算可以令人满意地匹配实验确定的取代苯(间位和对位)以及噻吩(α和β位置)中H / D交换活化的位置特异性Gibbs能量。
更新日期:2020-11-23
中文翻译:

Pt(II)CNN-Pincer配合物/ 2,2,2-三氟乙醇-d 1体系中取代(杂)芳烃的C(sp 2)-H键的催化氘代:取代基对反应速率和选择性的影响
已经在均相存在下,在涉及其C(sp 2)-H键和2,2,2-三氟乙醇-d 1(TFE- d 1)的催化H / D交换反应中测试了34种(杂)芳烃衍生物。Pt(II)配合物1在80°C受磺化CNN-钳夹配体支撑。18种底物,包括一种药物(萘普生),在1种存在下稳定它们在H / D交换反应中的活性已通过其特定位置的氘化程度以及在许多情况下的反应动力学选择性来表征。对于反应性最强的底物,氘的程度接近可交换H和D原子的预期统计分布:例如23小时后苯酚的67-69%和45分钟后吲哚β-CH键的88%。对于一些底物(N,N-二甲基苯胺,吲哚,硝基苯),H / D交换具有很高的位置选择性。没有令人满意的相关性被发现位置特异性的(间间,对位)H / d交换速率常数X单取代的苯和哈米特σ X常数。提出该观察结果与CH键活化的协调性质有关,CH键活化的速率决定了Pt(II)中心的CH键氧化加成。哈米特σ的一种新颖的规模中号X常数被引入来表征C(SP的反应2中的过渡金属介导的反应)-H键。使用DFT计算可以令人满意地匹配实验确定的取代苯(间位和对位)以及噻吩(α和β位置)中H / D交换活化的位置特异性Gibbs能量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号