当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of β-CF3 β-Amino Esters with an Indane Backbone by Rhenium-Catalyzed [3+2] Annulation
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.orglett.0c03239 Tingjun Hu 1 , Yuanqing Xu 1 , Saisai Zhang 1 , Heng-Ying Xiong 1 , Guangwu Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.orglett.0c03239 Tingjun Hu 1 , Yuanqing Xu 1 , Saisai Zhang 1 , Heng-Ying Xiong 1 , Guangwu Zhang 1
Affiliation
|
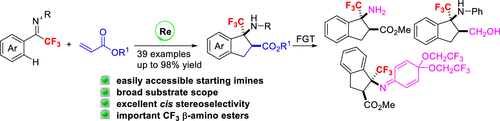
The [3+2] annulation of trifluoromethylated ketimines with acrylates has been enabled by rhenium-catalyzed C–H activation, delivering a variety of β-CF3 β-amino esters. The reaction has exhibited broad substrate generality regarding aromatic CF3-ketimines and acrylates, the ability for gram scale synthesis, and facile derivation of the annulation products. The transformation is one of the few examples in which challenging sp2 C–H bonds of CF3-ketimines have been functionalized. The rapid assembly of biologically important fluorinated β-amino esters by this strategy will benefit the related studies and inspire a new approach for fluorinated motif synthesis.
中文翻译:

的合成β-CF 3与由铼催化的二氢化茚骨干β氨基酯[3 + 2]环
三氟甲基化的酮亚胺与丙烯酸酯的[3 + 2]环已由铼催化C-H活化启用,提供各种β-CF的3 β氨基酯。该反应在芳族CF 3-酮亚胺和丙烯酸酯,克规模合成的能力以及环合产物的容易衍生方面表现出广泛的底物通用性。该转化是CF 3-酮亚胺的具有挑战性的sp 2 C–H键已被官能化的少数例子之一。通过这种策略快速组装具有生物学意义的重要氟化β-氨基酯将有益于相关研究,并启发了一种新的氟化基序合成方法。
更新日期:2020-11-21
中文翻译:

的合成β-CF 3与由铼催化的二氢化茚骨干β氨基酯[3 + 2]环
三氟甲基化的酮亚胺与丙烯酸酯的[3 + 2]环已由铼催化C-H活化启用,提供各种β-CF的3 β氨基酯。该反应在芳族CF 3-酮亚胺和丙烯酸酯,克规模合成的能力以及环合产物的容易衍生方面表现出广泛的底物通用性。该转化是CF 3-酮亚胺的具有挑战性的sp 2 C–H键已被官能化的少数例子之一。通过这种策略快速组装具有生物学意义的重要氟化β-氨基酯将有益于相关研究,并启发了一种新的氟化基序合成方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号