当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature Dependence of Protein Solution Viscosity and Protein–Protein Interactions: Insights into the Origins of High-Viscosity Protein Solutions
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.molpharmaceut.0c00552 Mahlet A Woldeyes 1 , Wei Qi 2 , Vladimir I Razinkov 2 , Eric M Furst 1 , Christopher J Roberts 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.molpharmaceut.0c00552 Mahlet A Woldeyes 1 , Wei Qi 2 , Vladimir I Razinkov 2 , Eric M Furst 1 , Christopher J Roberts 1
Affiliation
|
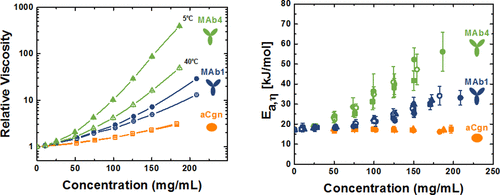
Protein solution viscosity (η) as a function of temperature was measured at a series of protein concentrations under a range of formulation conditions for two monoclonal antibodies (MAbs) and a globular protein (aCgn). Based on theoretical arguments, a strong temperature dependence for protein–protein interactions (PPI) indicates highly anisotropic, short-ranged attractions that could lead to higher solution viscosities. The semi-empirical Ross-Minton model was used to determine the apparent intrinsic viscosity, shape, and “crowding” factors for each protein as a function of temperature and formulation conditions. The apparent intrinsic viscosity was independent of temperature for aCgn, while a slight decrease with increasing temperature was observed for the MAbs. The temperature dependence of solution viscosity was analyzed using the Andrade-Eyring equation to determine the effective activation energy of viscous flow (Ea,η). While Ea,η values were different for each protein, they were independent of formulation conditions for a given protein. PPI were quantified via the osmotic second virial coefficient (B22) and the protein diffusion interaction parameter (kD) as a function of temperature under the same formulation conditions as the viscosity measurements. Net interactions ranged from strongly attractive to repulsive by changing formulation pH and ionic strength for each protein. Overall, larger activation energies for PPI corresponded to larger activation energies for η, and those were predictive of the highest η values at higher protein concentrations.
中文翻译:

蛋白质溶液粘度和蛋白质-蛋白质相互作用的温度依赖性:深入了解高粘度蛋白质溶液的起源
在两种单克隆抗体 (MAb) 和球状蛋白 (aCgn) 的一系列制剂条件下,在一系列蛋白质浓度下测量作为温度函数的蛋白质溶液粘度 (η)。基于理论论据,蛋白质-蛋白质相互作用 (PPI) 的强烈温度依赖性表明高度各向异性的短程吸引力可能导致更高的溶液粘度。半经验 Ross-Minton 模型用于确定作为温度和配方条件函数的每种蛋白质的表观特性粘度、形状和“拥挤”因子。aCgn 的表观特性粘度与温度无关,而 MAb 的表观特性粘度随温度升高而略有下降。E a,η )。虽然每种蛋白质的E a,η值不同,但它们与给定蛋白质的制剂条件无关。在与粘度测量相同的配方条件下,PPI 通过渗透第二维里系数 ( B 22 ) 和蛋白质扩散相互作用参数 ( k D ) 作为温度的函数进行量化。通过改变每种蛋白质的配方 pH 值和离子强度,净相互作用范围从强烈吸引到排斥。总体而言,PPI 的较大活化能对应于 η 的较大活化能,并且这些是在较高蛋白质浓度下最高 η 值的预测。
更新日期:2020-12-07
中文翻译:

蛋白质溶液粘度和蛋白质-蛋白质相互作用的温度依赖性:深入了解高粘度蛋白质溶液的起源
在两种单克隆抗体 (MAb) 和球状蛋白 (aCgn) 的一系列制剂条件下,在一系列蛋白质浓度下测量作为温度函数的蛋白质溶液粘度 (η)。基于理论论据,蛋白质-蛋白质相互作用 (PPI) 的强烈温度依赖性表明高度各向异性的短程吸引力可能导致更高的溶液粘度。半经验 Ross-Minton 模型用于确定作为温度和配方条件函数的每种蛋白质的表观特性粘度、形状和“拥挤”因子。aCgn 的表观特性粘度与温度无关,而 MAb 的表观特性粘度随温度升高而略有下降。E a,η )。虽然每种蛋白质的E a,η值不同,但它们与给定蛋白质的制剂条件无关。在与粘度测量相同的配方条件下,PPI 通过渗透第二维里系数 ( B 22 ) 和蛋白质扩散相互作用参数 ( k D ) 作为温度的函数进行量化。通过改变每种蛋白质的配方 pH 值和离子强度,净相互作用范围从强烈吸引到排斥。总体而言,PPI 的较大活化能对应于 η 的较大活化能,并且这些是在较高蛋白质浓度下最高 η 值的预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号