当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Local Targeting of Lung-Tumor-Associated Macrophages with Pulmonary Delivery of a CSF-1R Inhibitor for the Treatment of Breast Cancer Lung Metastases
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.molpharmaceut.0c00983 Sulaiman S Alhudaithi 1 , Rashed M Almuqbil 1 , Hanming Zhang 1 , Elizabeth R Bielski 1 , Wei Du 2 , Fatemah S Sunbul 1 , Paula D Bos 2, 3 , Sandro R P da Rocha 1, 3
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.molpharmaceut.0c00983 Sulaiman S Alhudaithi 1 , Rashed M Almuqbil 1 , Hanming Zhang 1 , Elizabeth R Bielski 1 , Wei Du 2 , Fatemah S Sunbul 1 , Paula D Bos 2, 3 , Sandro R P da Rocha 1, 3
Affiliation
|
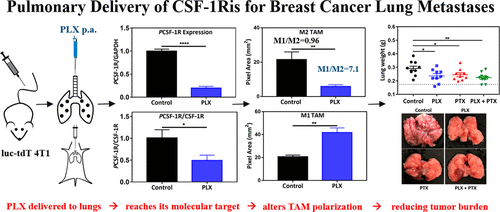
The lungs are major sites of metastases for several cancer types, including breast cancer (BC). Prognosis and quality of life of BC patients that develop pulmonary metastases are negatively impacted. The development of strategies to slow the growth and relieve the symptoms of BC lung metastases (BCLM) is thus an important goal in the management of BC. However, systemically administered first line small molecule chemotherapeutics have poor pharmacokinetic profiles and biodistribution to the lungs and significant off-target toxicity, severely compromising their effectiveness. In this work, we propose the local delivery of add-on immunotherapy to the lungs to support first line chemotherapy treatment of advanced BC. In a syngeneic murine model of BCLM, we show that local pulmonary administration (p.a.) of PLX-3397 (PLX), a colony-stimulating factor 1 receptor inhibitor (CSF-1Ri), is capable of overcoming physiological barriers of the lung epithelium, penetrating the tumor microenvironment (TME), and decreasing phosphorylation of CSF-1 receptors, as shown by the Western blot of lung tumor nodules. That inhibition is accompanied by an overall decrease in the abundance of protumorigenic (M2-like) macrophages in the TME, with a concomitant increase in the amount of antitumor (M1-like) macrophages when compared to the vehicle-treated control. These effects with PLX (p.a.) were achieved using a much smaller dose (1 mg/kg, every other day) compared to the systemic doses typically used in preclinical studies (40–800 mg/kg/day). As an additive in combination with intravenous (i.v.) administration of paclitaxel (PTX), PLX (p.a.) leads to a decrease in tumor burden without additional toxicity. These results suggested that the proposed immunochemotherapy, with regional pulmonary delivery of PLX along with the i.v. standard of care chemotherapy, may lead to new opportunities to improve treatment, quality of life, and survival of patients with BCLM.
中文翻译:

肺肿瘤相关巨噬细胞的肺肿瘤相关巨噬细胞的局部靶向与 CSF-1R 抑制剂的肺递送治疗乳腺癌肺转移
肺是多种癌症类型的主要转移部位,包括乳腺癌 (BC)。发生肺转移的 BC 患者的预后和生活质量受到负面影响。因此,制定减缓 BC 肺转移 (BCLM) 生长和缓解症状的策略是 BC 管理的重要目标。然而,全身给药的一线小分子化疗药物的药代动力学特征和肺部生物分布较差,并且具有显着的脱靶毒性,严重影响了其有效性。在这项工作中,我们建议将附加免疫疗法局部递送至肺部,以支持晚期 BC 的一线化疗治疗。在 BCLM 的同源小鼠模型中,我们显示 PLX-3397 (PLX) 的局部肺部给药 (pa),集落刺激因子 1 受体抑制剂 (CSF-1Ri),能够克服肺上皮的生理屏障,穿透肿瘤微环境 (TME),并降低 CSF-1 受体的磷酸化,如肺的蛋白质印迹所示肿瘤结节。这种抑制伴随着 TME 中促肿瘤(M2 样)巨噬细胞丰度的总体下降,与载体处理的对照相比,抗肿瘤(M1 样)巨噬细胞的数量随之增加。与临床前研究中通常使用的全身剂量(40-800 mg/kg/天)相比,PLX(pa)的这些效果使用更小的剂量(1 mg/kg,每隔一天)实现。作为添加剂与静脉注射 (iv) 紫杉醇 (PTX)、PLX (pa ) 导致肿瘤负荷减少而没有额外的毒性。这些结果表明,拟议的免疫化学疗法、PLX 的局部肺部递送以及 iv 护理化疗标准,可能会为改善 BCLM 患者的治疗、生活质量和生存带来新的机会。
更新日期:2020-12-07
中文翻译:

肺肿瘤相关巨噬细胞的肺肿瘤相关巨噬细胞的局部靶向与 CSF-1R 抑制剂的肺递送治疗乳腺癌肺转移
肺是多种癌症类型的主要转移部位,包括乳腺癌 (BC)。发生肺转移的 BC 患者的预后和生活质量受到负面影响。因此,制定减缓 BC 肺转移 (BCLM) 生长和缓解症状的策略是 BC 管理的重要目标。然而,全身给药的一线小分子化疗药物的药代动力学特征和肺部生物分布较差,并且具有显着的脱靶毒性,严重影响了其有效性。在这项工作中,我们建议将附加免疫疗法局部递送至肺部,以支持晚期 BC 的一线化疗治疗。在 BCLM 的同源小鼠模型中,我们显示 PLX-3397 (PLX) 的局部肺部给药 (pa),集落刺激因子 1 受体抑制剂 (CSF-1Ri),能够克服肺上皮的生理屏障,穿透肿瘤微环境 (TME),并降低 CSF-1 受体的磷酸化,如肺的蛋白质印迹所示肿瘤结节。这种抑制伴随着 TME 中促肿瘤(M2 样)巨噬细胞丰度的总体下降,与载体处理的对照相比,抗肿瘤(M1 样)巨噬细胞的数量随之增加。与临床前研究中通常使用的全身剂量(40-800 mg/kg/天)相比,PLX(pa)的这些效果使用更小的剂量(1 mg/kg,每隔一天)实现。作为添加剂与静脉注射 (iv) 紫杉醇 (PTX)、PLX (pa ) 导致肿瘤负荷减少而没有额外的毒性。这些结果表明,拟议的免疫化学疗法、PLX 的局部肺部递送以及 iv 护理化疗标准,可能会为改善 BCLM 患者的治疗、生活质量和生存带来新的机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号