Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Competition between Conversion Reaction with Cerium Dioxide and Lithium Plating in Superconcentrated Electrolyte
Langmuir ( IF 3.9 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.langmuir.0c02622 Tohru Shiga 1 , Yumi Masuoka 1 , Yuichi Kato 1
Langmuir ( IF 3.9 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.langmuir.0c02622 Tohru Shiga 1 , Yumi Masuoka 1 , Yuichi Kato 1
Affiliation
|
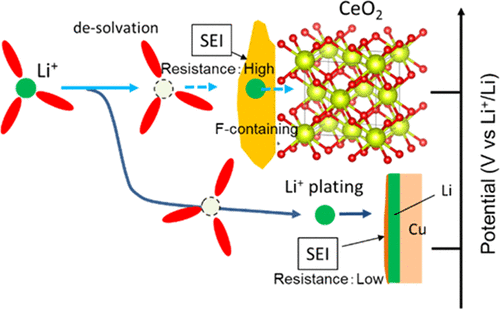
Li-ion insertion into cerium dioxide (CeO2) and its subsequent conversion reaction were studied using a CeO2/copper composite electrode in a superconcentrated electrolyte of lithium bis(fluorosulfonyl)amide (LiFSA) and methylphenylamino-di(trifluoroethyl) phosphate (PNMePh) under conditions promoting Li plating/stripping. Since the conversion reaction potential with CeO2 generally lies above the Li plating/stripping level, the conversion ideally occurs first in the cathodic scan. However, the conversion reaction was delayed until after the Li plating in the superconcentrated electrolyte contrary to expectations, whereas this phenomenon was unobserved in a dilute LiFSA/PNMePh electrolyte. Energy-dispersive X-ray spectroscopy and electrochemical impedance analysis indicated that the reversed order of the electrochemical behaviors was caused by the solid electrolyte interphase (SEI) on the CeO2, which had a different material composition and a higher interfacial resistance than the SEI on electrodeposited metallic lithium.
中文翻译:

超浓缩电解质中二氧化铈转化反应与镀锂之间的竞争
使用CeO 2 /铜复合电极在双(氟磺酰基)酰胺锂(LiFSA)和甲基苯基氨基-二(三氟乙基)磷酸酯(PNMePh)的超浓缩电解质中研究了锂离子插入二氧化铈(CeO 2)中及其后续的转化反应)在促进锂电镀/剥离的条件下。由于与CeO 2的转化反应电位由于通常位于Li镀层/剥离层之上,因此理想情况下首先在阴极扫描中进行转换。然而,转化反应被推迟到超浓缩电解质中的镀锂之后,这与预期相反,而这种现象在稀LiFSA / PNMePh电解质中未观察到。能量色散X射线能谱和电化学阻抗分析表明,电化学行为的逆序是由CeO 2上的固体电解质相(SEI)引起的,该材料具有不同的材料组成和更高的界面电阻。电沉积的金属锂。
更新日期:2020-11-25
中文翻译:

超浓缩电解质中二氧化铈转化反应与镀锂之间的竞争
使用CeO 2 /铜复合电极在双(氟磺酰基)酰胺锂(LiFSA)和甲基苯基氨基-二(三氟乙基)磷酸酯(PNMePh)的超浓缩电解质中研究了锂离子插入二氧化铈(CeO 2)中及其后续的转化反应)在促进锂电镀/剥离的条件下。由于与CeO 2的转化反应电位由于通常位于Li镀层/剥离层之上,因此理想情况下首先在阴极扫描中进行转换。然而,转化反应被推迟到超浓缩电解质中的镀锂之后,这与预期相反,而这种现象在稀LiFSA / PNMePh电解质中未观察到。能量色散X射线能谱和电化学阻抗分析表明,电化学行为的逆序是由CeO 2上的固体电解质相(SEI)引起的,该材料具有不同的材料组成和更高的界面电阻。电沉积的金属锂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号