当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Iron-Catalyzed Cross-[4+4]-Cycloaddition of 1,3-Dienes Provides Chiral Cyclooctadienes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-11 , DOI: 10.1021/jacs.0c09486 Elena Braconi 1 , Alissa C. Götzinger 1 , Nicolai Cramer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-11 , DOI: 10.1021/jacs.0c09486 Elena Braconi 1 , Alissa C. Götzinger 1 , Nicolai Cramer 1
Affiliation
|
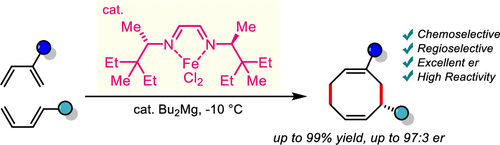
Chiral cyclooctadienes are a frequently occurring scaffold in natural products and specialty chemicals, and are used as ligands in asymmetric catalysis. Accessing substituted cyclooctadienes in an efficient asymmetric fashion has been notoriously challenging. We report an iron-catalyzed enantioselective cross-[4+4]-cycloaddition of 1,3-dienes to form substituted cyclooctadienes under very mild conditions. A highly tailored chiral α-diimine iron complex is key for the success of the transformation providing a balanced performance between reactivity, excellent cross-selectivity and very high enantioselectivity. Steric maps of the complexes help accounting for the observed selectivity. The developed method allows rapid and atom-economic access to novel differently functionalized cyclooctadienes in very high yields and enantioselectivities.
中文翻译:

1,3-二烯的对映选择性铁催化交叉[4+4]-环加成提供手性环辛二烯
手性环辛二烯是天然产物和特种化学品中经常出现的支架,在不对称催化中用作配体。以有效的不对称方式获得取代的环辛二烯一直是众所周知的挑战。我们报告了一种铁催化的对映选择性交叉 [4+4]-环加成反应,在非常温和的条件下,1,3-二烯形成取代的环辛二烯。高度定制的手性 α-二亚胺铁配合物是转化成功的关键,可在反应性、优异的交叉选择性和非常高的对映选择性之间提供平衡的性能。复合物的立体图有助于解释观察到的选择性。所开发的方法允许以非常高的产率和对映选择性快速和原子经济地获得新的不同官能化的环辛二烯。
更新日期:2020-11-11
中文翻译:

1,3-二烯的对映选择性铁催化交叉[4+4]-环加成提供手性环辛二烯
手性环辛二烯是天然产物和特种化学品中经常出现的支架,在不对称催化中用作配体。以有效的不对称方式获得取代的环辛二烯一直是众所周知的挑战。我们报告了一种铁催化的对映选择性交叉 [4+4]-环加成反应,在非常温和的条件下,1,3-二烯形成取代的环辛二烯。高度定制的手性 α-二亚胺铁配合物是转化成功的关键,可在反应性、优异的交叉选择性和非常高的对映选择性之间提供平衡的性能。复合物的立体图有助于解释观察到的选择性。所开发的方法允许以非常高的产率和对映选择性快速和原子经济地获得新的不同官能化的环辛二烯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号