当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper(I)-Catalyzed Asymmetric 1,4-Conjugate Hydrophosphination of α,β-Unsaturated Amides
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-11-11 , DOI: 10.1021/jacs.0c09654 Yan-Bo Li 1 , Hu Tian 1 , Liang Yin 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-11-11 , DOI: 10.1021/jacs.0c09654 Yan-Bo Li 1 , Hu Tian 1 , Liang Yin 1
Affiliation
|
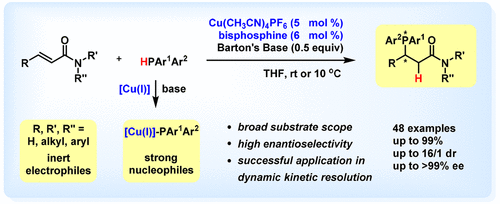
A catalytic asymmetric conjugate hydrophosphination of α,β-unsaturated amides is accomplished by virtue of the strong nucleophilicity of copper(I)-PPh2 species, which provides an array of chiral phosphines bearing an amide moiety in high to excellent yields with excellent enantioselectivity. Furthermore, the dynamic kinetic resolution of unsymmetrical diarylphosphines (HPAr1Ar2) is successfully carried out through the copper(I)-catalyzed conjugate addition to α,β-unsaturated amides, which affords P-chiral phosphines with good-to-high diastereoselectivity and high enantioselectivity. 1H NMR studies show that the precoordination of HPPh2 to copper(I)-bisphosphine complex is critical for the efficient deprotonation by Barton's Base. Moreover, the relative stability of the copper(I)-(R,RP)-TANIAPHOS complex in the presence of excessive HPPh2, confirmed by 31P NMR studies, is pivotal for the high asymmetric induction, as the ligand exchange between bisphosphine and HPPh2 would significantly reduce the enantioselectivity. At last, a double catalytic asymmetric conjugate hydrophosphination furnishes the corresponding product in high yield with high diastereoselectivity and excellent enantioselectivity, which is transformed to a chiral pincer palladium complex in moderate yield. This chiral palladium complex is demonstrated as an excellent catalyst in the asymmetric conjugate hydrophosphination of chalcone.
中文翻译:

铜(I)-催化的α,β-不饱和酰胺的不对称1,4-共轭氢膦化反应
α,β-不饱和酰胺的催化不对称共轭氢膦化反应是通过铜 (I)-PPh2 物质的强亲核性完成的,它提供了一系列带有酰胺部分的手性膦,收率高到极好,具有极好的对映选择性。此外,不对称二芳基膦(HPAr1Ar2)的动态动力学拆分是通过铜(I)催化的共轭加成与α,β-不饱和酰胺成功进行的,这提供了具有良好到高非对映选择性和高对映选择性的P-手性膦. 1H NMR 研究表明,HPPh2 与铜 (I)-双膦配合物的预配位对于巴顿碱的有效去质子化至关重要。此外,在过量 HPPh2 存在下,铜 (I)-(R,RP)-TANIAPHOS 络合物的相对稳定性,由 31P NMR 研究证实,对于高不对称诱导至关重要,因为双膦和 HPPh2 之间的配体交换会显着降低对映选择性。最后,双催化不对称共轭氢膦化反应以高产率提供相应产物,具有高非对映选择性和优异的对映选择性,并以中等产率转化为手性钳形钯配合物。这种手性钯配合物在查耳酮的不对称共轭氢膦化反应中被证明是一种极好的催化剂。双催化不对称共轭氢膦化反应以高收率提供相应产物,具有高非对映选择性和优异的对映选择性,其以中等收率转化为手性钳形钯配合物。这种手性钯配合物在查耳酮的不对称共轭氢膦化反应中被证明是一种极好的催化剂。双催化不对称共轭氢膦化反应以高产率提供相应产物,具有高非对映选择性和优异的对映选择性,其以中等产率转化为手性钳形钯配合物。这种手性钯配合物在查耳酮的不对称共轭氢膦化反应中被证明是一种极好的催化剂。
更新日期:2020-11-11
中文翻译:

铜(I)-催化的α,β-不饱和酰胺的不对称1,4-共轭氢膦化反应
α,β-不饱和酰胺的催化不对称共轭氢膦化反应是通过铜 (I)-PPh2 物质的强亲核性完成的,它提供了一系列带有酰胺部分的手性膦,收率高到极好,具有极好的对映选择性。此外,不对称二芳基膦(HPAr1Ar2)的动态动力学拆分是通过铜(I)催化的共轭加成与α,β-不饱和酰胺成功进行的,这提供了具有良好到高非对映选择性和高对映选择性的P-手性膦. 1H NMR 研究表明,HPPh2 与铜 (I)-双膦配合物的预配位对于巴顿碱的有效去质子化至关重要。此外,在过量 HPPh2 存在下,铜 (I)-(R,RP)-TANIAPHOS 络合物的相对稳定性,由 31P NMR 研究证实,对于高不对称诱导至关重要,因为双膦和 HPPh2 之间的配体交换会显着降低对映选择性。最后,双催化不对称共轭氢膦化反应以高产率提供相应产物,具有高非对映选择性和优异的对映选择性,并以中等产率转化为手性钳形钯配合物。这种手性钯配合物在查耳酮的不对称共轭氢膦化反应中被证明是一种极好的催化剂。双催化不对称共轭氢膦化反应以高收率提供相应产物,具有高非对映选择性和优异的对映选择性,其以中等收率转化为手性钳形钯配合物。这种手性钯配合物在查耳酮的不对称共轭氢膦化反应中被证明是一种极好的催化剂。双催化不对称共轭氢膦化反应以高产率提供相应产物,具有高非对映选择性和优异的对映选择性,其以中等产率转化为手性钳形钯配合物。这种手性钯配合物在查耳酮的不对称共轭氢膦化反应中被证明是一种极好的催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号