当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibrium of MnSO4–MgSO4–H2O and (NH4)2SO4–MnSO4–MgSO4–H2O Systems at T = 303.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.jced.0c00789 Manxin Ding 1 , Yujia Zhang 1 , Yongsheng Ren 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.jced.0c00789 Manxin Ding 1 , Yujia Zhang 1 , Yongsheng Ren 1
Affiliation
|
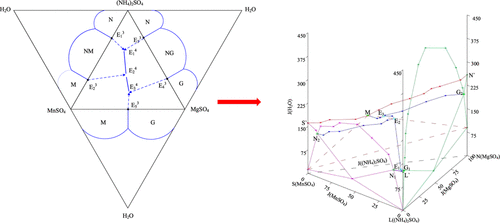
In this study, the stable (solid + liquid) phase equilibrium and physical–chemical properties of the ternary MnSO4–MgSO4–H2O system at T = 303.15 K have been determined by the method of isothermal solution saturation. It is found that there are three crystalline regions, one unsaturated crystallization region, and one invariant point. The density and refractive index of this system were compared with the experimental values, and it is found that there is little difference between the two systems. The experimental solubility data were compared with the literature values, and the invariant points at different temperatures were fitted to provide data for the water-salt system containing manganese sulfate and magnesium sulfate. At the same time, the solubility data of the MnSO4–MgSO4–(NH4)2SO4–H2O system were determined by the same method, and the phase diagram was drawn. Comparing the actual phase diagram measured by the experiment with the predicted phase diagram obtained by means of translation method, it is found that they have the same crystallization regions. In order to see more clearly the relationship between the quaternary MnSO4–MgSO4–(NH4)2SO4–H2O system and its three ternary sub-systems (MnSO4–(NH4)2SO4–H2O, MgSO4–(NH4)2SO4–H2O, and MnSO4–MgSO4–H2O), we have plotted the related stereoscopic phase diagram. The research of this study provides the data basis for the crystallization process of manganese sulfate.
中文翻译:

在T = 303.15 K时MnSO 4 –MgSO 4 –H 2 O和(NH 4)2 SO 4 –MnSO 4 –MgSO 4 –H 2 O系统的固液平衡。
在这项研究中,三元MnSO 4 –MgSO 4 –H 2 O系统在T时具有稳定的(固+液相)相平衡和物理化学性质。通过等温溶液饱和法确定了303.15K。发现存在三个结晶区,一个不饱和结晶区和一个不变点。将该系统的密度和折射率与实验值进行比较,发现这两个系统之间几乎没有差异。将实验溶解度数据与文献值进行了比较,并拟合了不同温度下的不变点,以提供包含硫酸锰和硫酸镁的水盐体系的数据。同时,MnSO 4 -MgSO 4-(NH 4)2 SO 4 -H 2的溶解度数据用相同的方法确定O体系,并绘制相图。将实验测得的实际相图与通过平移法获得的预测相图进行比较,发现它们具有相同的结晶区。为了更清楚地了解四元MnSO 4 -MgSO 4-(NH 4)2 SO 4 -H 2 O系统及其三个三元子系统(MnSO 4-(NH 4)2 SO 4 -H 2 O,MgSO 4 –(NH 4)2 SO 4 –H2 O和MnSO 4 -MgSO 4 -H 2 O),我们已经绘制了相关的立体相图。本研究为硫酸锰的结晶过程提供了数据基础。
更新日期:2021-01-14
中文翻译:

在T = 303.15 K时MnSO 4 –MgSO 4 –H 2 O和(NH 4)2 SO 4 –MnSO 4 –MgSO 4 –H 2 O系统的固液平衡。
在这项研究中,三元MnSO 4 –MgSO 4 –H 2 O系统在T时具有稳定的(固+液相)相平衡和物理化学性质。通过等温溶液饱和法确定了303.15K。发现存在三个结晶区,一个不饱和结晶区和一个不变点。将该系统的密度和折射率与实验值进行比较,发现这两个系统之间几乎没有差异。将实验溶解度数据与文献值进行了比较,并拟合了不同温度下的不变点,以提供包含硫酸锰和硫酸镁的水盐体系的数据。同时,MnSO 4 -MgSO 4-(NH 4)2 SO 4 -H 2的溶解度数据用相同的方法确定O体系,并绘制相图。将实验测得的实际相图与通过平移法获得的预测相图进行比较,发现它们具有相同的结晶区。为了更清楚地了解四元MnSO 4 -MgSO 4-(NH 4)2 SO 4 -H 2 O系统及其三个三元子系统(MnSO 4-(NH 4)2 SO 4 -H 2 O,MgSO 4 –(NH 4)2 SO 4 –H2 O和MnSO 4 -MgSO 4 -H 2 O),我们已经绘制了相关的立体相图。本研究为硫酸锰的结晶过程提供了数据基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号