当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macro and Molecular Level Insights on Gas Hydrate Growth in the Presence of Hofmeister Salts
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.iecr.0c04389 Nilesh Choudhary 1, 2 , Omkar Singh Kushwaha 1 , Gaurav Bhattacharjee 1 , Suman Chakrabarty 3 , Rajnish Kumar 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.iecr.0c04389 Nilesh Choudhary 1, 2 , Omkar Singh Kushwaha 1 , Gaurav Bhattacharjee 1 , Suman Chakrabarty 3 , Rajnish Kumar 1
Affiliation
|
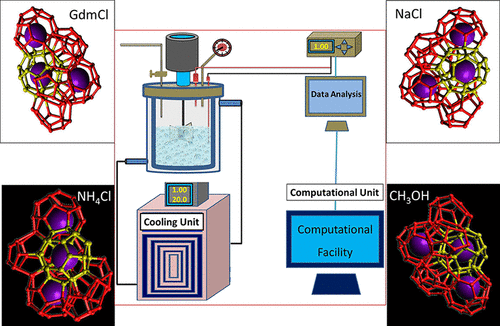
The effect of few monovalent salts (NaCl, NH4Cl, and GdmCl) as additives, according to the Hofmeister series on the growth of methane gas hydrates, has been studied using experiments as well as molecular dynamics (MD) simulation. Further, the Hofmeister effects on hydrate crystallization have been correlated with the methanol as an additive, which is a known thermodynamic hydrate inhibitor for hydrate growth. One of the previous studies (discussed later in this article) available in the literature concludes that methane hydrate formation from ice might show enhanced kinetics in the presence of salts; this behavior is contrary to the general usage of such salts as hydrate inhibitors. This conclusion may not necessarily be true for experiments done with liquid water, and therefore, this work explores the behavior of these salts in a lab-scale setup. In addition, current work reports detailed MD simulation studies to gain insight into the mechanism of hydrate formation in the presence of Hofmeister series salts at two different concentrations of 1 and 10 wt % in water and compare the results with hydrate formation in the methanol–water system. Our study suggests that the presence of these additives at low concentrations (1 wt %) does enhance the hydrate growth kinetics. However, at higher concentrations (10 wt %), inhibition by these additives persisted, and prolonged nucleation, as well as retarded growth, was observed.
中文翻译:

霍夫迈斯特盐存在下天然气水合物生长的宏观和分子水平洞察
少数一价盐(NaCl,NH 4根据关于甲烷气体水合物生长的Hofmeister系列,已使用实验和分子动力学(MD)模拟研究了作为添加剂的Cl和GdmCl)。此外,霍夫迈斯特对水合物结晶的影响已经与作为添加剂的甲醇相关联,甲醇是已知的用于水合物生长的热力学水合物抑制剂。文献中已有的一项先前的研究(在本文的下文中讨论)得出的结论是,在盐的存在下,冰中甲烷水合物的形成可能显示出增强的动力学。这种行为与这类盐作为水合物抑制剂的一般用法相反。对于使用液态水进行的实验,该结论可能不一定是正确的,因此,这项工作探索了实验室规模设置中这些盐的行为。此外,当前的工作报告详细进行了MD模拟研究,以了解在两种不同浓度的Hofmeister系列盐在水中的浓度为1和10 wt%时水合物形成的机理,并将结果与甲醇-水系统中水合物的形成进行比较。我们的研究表明,低浓度(1 wt%)的这些添加剂的存在确实增强了水合物的生长动力学。然而,在更高的浓度(10wt%)下,这些添加剂的抑制作用持续存在,并且观察到延长的成核作用以及生长延迟。我们的研究表明,低浓度(1 wt%)的这些添加剂的存在确实增强了水合物的生长动力学。然而,在更高的浓度(10wt%)下,这些添加剂的抑制作用持续存在,并且观察到延长的成核作用以及生长延迟。我们的研究表明,低浓度(1 wt%)的这些添加剂的存在确实增强了水合物的生长动力学。然而,在更高的浓度(10wt%)下,这些添加剂的抑制作用持续存在,并且观察到延长的成核作用以及生长延迟。
更新日期:2020-11-25
中文翻译:

霍夫迈斯特盐存在下天然气水合物生长的宏观和分子水平洞察
少数一价盐(NaCl,NH 4根据关于甲烷气体水合物生长的Hofmeister系列,已使用实验和分子动力学(MD)模拟研究了作为添加剂的Cl和GdmCl)。此外,霍夫迈斯特对水合物结晶的影响已经与作为添加剂的甲醇相关联,甲醇是已知的用于水合物生长的热力学水合物抑制剂。文献中已有的一项先前的研究(在本文的下文中讨论)得出的结论是,在盐的存在下,冰中甲烷水合物的形成可能显示出增强的动力学。这种行为与这类盐作为水合物抑制剂的一般用法相反。对于使用液态水进行的实验,该结论可能不一定是正确的,因此,这项工作探索了实验室规模设置中这些盐的行为。此外,当前的工作报告详细进行了MD模拟研究,以了解在两种不同浓度的Hofmeister系列盐在水中的浓度为1和10 wt%时水合物形成的机理,并将结果与甲醇-水系统中水合物的形成进行比较。我们的研究表明,低浓度(1 wt%)的这些添加剂的存在确实增强了水合物的生长动力学。然而,在更高的浓度(10wt%)下,这些添加剂的抑制作用持续存在,并且观察到延长的成核作用以及生长延迟。我们的研究表明,低浓度(1 wt%)的这些添加剂的存在确实增强了水合物的生长动力学。然而,在更高的浓度(10wt%)下,这些添加剂的抑制作用持续存在,并且观察到延长的成核作用以及生长延迟。我们的研究表明,低浓度(1 wt%)的这些添加剂的存在确实增强了水合物的生长动力学。然而,在更高的浓度(10wt%)下,这些添加剂的抑制作用持续存在,并且观察到延长的成核作用以及生长延迟。











































 京公网安备 11010802027423号
京公网安备 11010802027423号