当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Mechanism of Self-Assembly by a Hydrogel-Forming Peptide
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.biomac.0c00989 Gabriel A Braun 1, 2 , Beatrice E Ary 1 , Alexander J Dear 3, 4 , Matthew C H Rohn 1 , Abigail M Payson 1 , David S M Lee 1 , Robert C Parry 1 , Connie Friedman 1 , Tuomas P J Knowles 3, 5 , Sara Linse 2 , Karin S Åkerfeldt 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.biomac.0c00989 Gabriel A Braun 1, 2 , Beatrice E Ary 1 , Alexander J Dear 3, 4 , Matthew C H Rohn 1 , Abigail M Payson 1 , David S M Lee 1 , Robert C Parry 1 , Connie Friedman 1 , Tuomas P J Knowles 3, 5 , Sara Linse 2 , Karin S Åkerfeldt 1
Affiliation
|
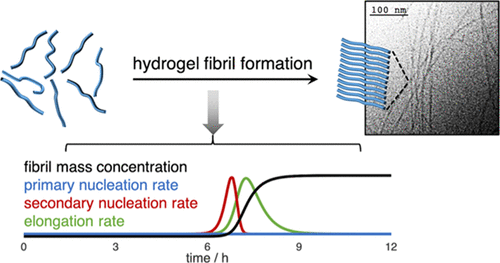
Self-assembling peptide-based hydrogels are a class of tunable soft materials that have been shown to be highly useful for a number of biomedical applications. The dynamic formation of the supramolecular fibrils that compose these materials has heretofore remained poorly characterized. A better understanding of this process would provide important insights into the behavior of these systems and could aid in the rational design of new peptide hydrogels. Here, we report the determination of the microscopic steps that underpin the self-assembly of a hydrogel-forming peptide, SgI37-49. Using theoretical models of linear polymerization to analyze the kinetic self-assembly data, we show that SgI37-49 fibril formation is driven by fibril-catalyzed secondary nucleation and that all the microscopic processes involved in SgI37-49 self-assembly display an enzyme-like saturation behavior. Moreover, this analysis allows us to quantify the rates of the underlying processes at different peptide concentrations and to calculate the time evolution of these reaction rates over the time course of self-assembly. We demonstrate here a new mechanistic approach for the study of self-assembling hydrogel-forming peptides, which is complementary to commonly used materials science characterization techniques.
中文翻译:

关于水凝胶形成肽自组装的机理
自组装的基于肽的水凝胶是一类可调节的柔软材料,已被证明对许多生物医学应用非常有用。迄今为止,组成这些材料的超分子原纤维的动态形成仍然不充分。对该过程的更好理解将为这些系统的行为提供重要的见解,并有助于新肽水凝胶的合理设计。在这里,我们报告了确定水凝胶形成肽SgI 37-49自组装的微观步骤的确定。使用线性聚合反应的理论模型分析动力学自组装数据,我们发现SgI 37-49原纤维催化的二次成核作用驱动原纤维形成,并且参与SgI 37-49自组装的所有微观过程都显示出类似酶的饱和行为。而且,这种分析使我们能够量化不同肽浓度下的潜在过程的速率,并计算这些反应速率在自组装过程中的时间演变。我们在这里展示了一种新的机制,用于研究自组装水凝胶形成肽,这是对常用材料科学表征技术的补充。
更新日期:2020-12-14
中文翻译:

关于水凝胶形成肽自组装的机理
自组装的基于肽的水凝胶是一类可调节的柔软材料,已被证明对许多生物医学应用非常有用。迄今为止,组成这些材料的超分子原纤维的动态形成仍然不充分。对该过程的更好理解将为这些系统的行为提供重要的见解,并有助于新肽水凝胶的合理设计。在这里,我们报告了确定水凝胶形成肽SgI 37-49自组装的微观步骤的确定。使用线性聚合反应的理论模型分析动力学自组装数据,我们发现SgI 37-49原纤维催化的二次成核作用驱动原纤维形成,并且参与SgI 37-49自组装的所有微观过程都显示出类似酶的饱和行为。而且,这种分析使我们能够量化不同肽浓度下的潜在过程的速率,并计算这些反应速率在自组装过程中的时间演变。我们在这里展示了一种新的机制,用于研究自组装水凝胶形成肽,这是对常用材料科学表征技术的补充。











































 京公网安备 11010802027423号
京公网安备 11010802027423号