当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Yeast Chd1p Unwraps the Exit Side DNA upon ATP Binding to Facilitate the Nucleosome Translocation Occurring upon ATP Hydrolysis
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.biochem.0c00747 Jaewon Kirk 1 , Ju Yeon Lee 1 , Yejin Lee 2 , Chanshin Kang 1 , Soochul Shin 1 , Eunhye Lee 2 , Ji-Joon Song 2 , Sungchul Hohng 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.biochem.0c00747 Jaewon Kirk 1 , Ju Yeon Lee 1 , Yejin Lee 2 , Chanshin Kang 1 , Soochul Shin 1 , Eunhye Lee 2 , Ji-Joon Song 2 , Sungchul Hohng 1
Affiliation
|
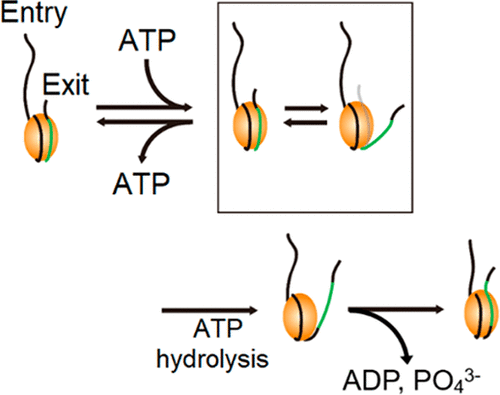
Chromodomain-helicase-DNA-binding protein 1 (CHD1) remodels chromatin by translocating nucleosomes along DNA, but its mechanism remains poorly understood. We use single-molecule fluorescence experiments to clarify the mechanism by which yeast CHD1 (Chd1p) remodels nucleosomes. We find that binding of ATP to Chd1p induces transient unwrapping of the DNA on the exit side of the nucleosome, facilitating nucleosome translocation. ATP hydrolysis is required to induce nucleosome translocation. The unwrapped DNA after translocation is then rewrapped after the release of the hydrolyzed nucleotide and phosphate, revealing that each step of the ATP hydrolysis cycle is responsible for a distinct step of nucleosome remodeling. These results show that Chd1p remodels nucleosomes via a mechanism that is unique among the other ATP-dependent chromatin remodelers.
中文翻译:

酵母Chd1p解开ATP结合后的出口侧DNA,以促进ATP水解后发生的核小体移位。
染色体域-解旋酶-DNA结合蛋白1(CHD1)通过沿DNA转移核小体来重塑染色质,但其机理仍知之甚少。我们使用单分子荧光实验来阐明酵母CHD1(Chd1p)重塑核小体的机制。我们发现,ATP与Chd1p的结合会诱导核小体出口侧DNA的瞬时解缠,从而促进核小体移位。ATP水解是诱导核小体转运所必需的。易位后解开的DNA然后在释放水解的核苷酸和磷酸盐后被重新包裹,这表明ATP水解循环的每个步骤都负责核小体重塑的不同步骤。这些结果表明,Chd1p通过其他ATP依赖的染色质重塑剂中独特的机制重塑核小体。
更新日期:2020-12-01
中文翻译:

酵母Chd1p解开ATP结合后的出口侧DNA,以促进ATP水解后发生的核小体移位。
染色体域-解旋酶-DNA结合蛋白1(CHD1)通过沿DNA转移核小体来重塑染色质,但其机理仍知之甚少。我们使用单分子荧光实验来阐明酵母CHD1(Chd1p)重塑核小体的机制。我们发现,ATP与Chd1p的结合会诱导核小体出口侧DNA的瞬时解缠,从而促进核小体移位。ATP水解是诱导核小体转运所必需的。易位后解开的DNA然后在释放水解的核苷酸和磷酸盐后被重新包裹,这表明ATP水解循环的每个步骤都负责核小体重塑的不同步骤。这些结果表明,Chd1p通过其他ATP依赖的染色质重塑剂中独特的机制重塑核小体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号