当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyamines Mediate Folding of Primordial Hyperacidic Helical Proteins
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.biochem.0c00800 Dragana Despotović 1 , Liam M Longo 1, 2, 3 , Einav Aharon 1 , Amit Kahana 1, 4 , Tali Scherf 5 , Ita Gruic-Sovulj 6 , Dan S Tawfik 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.biochem.0c00800 Dragana Despotović 1 , Liam M Longo 1, 2, 3 , Einav Aharon 1 , Amit Kahana 1, 4 , Tali Scherf 5 , Ita Gruic-Sovulj 6 , Dan S Tawfik 1
Affiliation
|
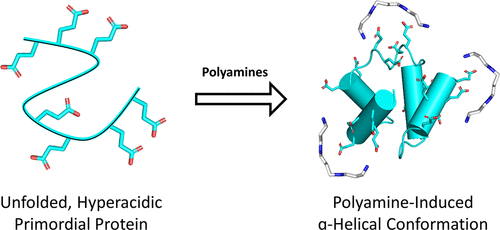
Polyamines are known to mediate diverse biological processes, and specifically to bind and stabilize compact conformations of nucleic acids, acting as chemical chaperones that promote folding by offsetting the repulsive negative charges of the phosphodiester backbone. However, whether and how polyamines modulate the structure and function of proteins remain unclear. In particular, early proteins are thought to have been highly acidic, like nucleic acids, due to a scarcity of basic amino acids in the prebiotic context. Perhaps polyamines, the abiotic synthesis of which is simple, could have served as chemical chaperones for such primordial proteins? We replaced all lysines of an ancestral 60-residue helix-bundle protein with glutamate, resulting in a disordered protein with 21 glutamates in total. Polyamines efficiently induce folding of this hyperacidic protein at submillimolar concentrations, and their potency scaled with the number of amine groups. Compared to cations, polyamines were several orders of magnitude more potent than Na+, while Mg2+ and Ca2+ had an effect similar to that of a diamine, inducing folding at approximately seawater concentrations. We propose that (i) polyamines and dications may have had a role in promoting folding of early proteins devoid of basic residues and (ii) coil–helix transitions could be the basis of polyamine regulation in contemporary proteins.
中文翻译:

多胺介导原始高酸性螺旋蛋白的折叠
已知多胺可介导多种生物过程,特别是结合和稳定核酸的紧凑构象,充当化学伴侣,通过抵消磷酸二酯主链的排斥性负电荷来促进折叠。然而,多胺是否以及如何调节蛋白质的结构和功能仍不清楚。特别是,由于在生命起源之前缺乏碱性氨基酸,早期蛋白质被认为像核酸一样具有高酸性。也许非生物合成很简单的多胺可以作为这种原始蛋白质的化学伴侣?我们用谷氨酸取代了祖先的 60 个残基螺旋束蛋白的所有赖氨酸,产生了总共 21 个谷氨酸的无序蛋白质。多胺在亚毫摩尔浓度下有效诱导这种高酸性蛋白质的折叠,并且其效力随着胺基的数量而变化。与阳离子相比,多胺比 Na +强几个数量级,而 Mg 2+和 Ca 2+具有与二胺类似的效果,在大约海水浓度下诱导折叠。我们提出,(i)多胺和二价阳离子可能在促进不含碱性残基的早期蛋白质折叠方面发挥作用,(ii)卷曲-螺旋转变可能是当代蛋白质中多胺调节的基础。
更新日期:2020-11-25
中文翻译:

多胺介导原始高酸性螺旋蛋白的折叠
已知多胺可介导多种生物过程,特别是结合和稳定核酸的紧凑构象,充当化学伴侣,通过抵消磷酸二酯主链的排斥性负电荷来促进折叠。然而,多胺是否以及如何调节蛋白质的结构和功能仍不清楚。特别是,由于在生命起源之前缺乏碱性氨基酸,早期蛋白质被认为像核酸一样具有高酸性。也许非生物合成很简单的多胺可以作为这种原始蛋白质的化学伴侣?我们用谷氨酸取代了祖先的 60 个残基螺旋束蛋白的所有赖氨酸,产生了总共 21 个谷氨酸的无序蛋白质。多胺在亚毫摩尔浓度下有效诱导这种高酸性蛋白质的折叠,并且其效力随着胺基的数量而变化。与阳离子相比,多胺比 Na +强几个数量级,而 Mg 2+和 Ca 2+具有与二胺类似的效果,在大约海水浓度下诱导折叠。我们提出,(i)多胺和二价阳离子可能在促进不含碱性残基的早期蛋白质折叠方面发挥作用,(ii)卷曲-螺旋转变可能是当代蛋白质中多胺调节的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号