当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Magnetically recoverable copper ferrite catalyzed cascade synthesis of 1,3‐dimethyl‐6‐nitro‐5‐arylpyrido[2,3‐d]pyrimidine‐2,4(1H,3H)‐diones under microwave irradiation and solvent‐less condition
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-11-10 , DOI: 10.1002/aoc.6091 Amar Jyoti Bhuyan 1 , Pubanita Bhuyan 1 , Bornali Boruah 2 , Lakhinath Saikia 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-11-10 , DOI: 10.1002/aoc.6091 Amar Jyoti Bhuyan 1 , Pubanita Bhuyan 1 , Bornali Boruah 2 , Lakhinath Saikia 1
Affiliation
|
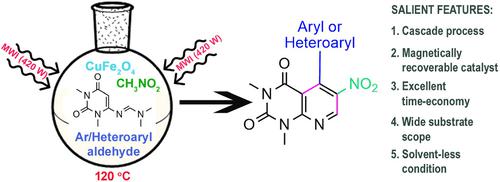
In recent years, magnetically active CuFe2O4 nanoparticles have been gaining significant interest in the field of heterogeneous catalysis as those can be easily prepared and effortlessly recovered from a reaction system. Here, we are reporting our work on cascade syntheses of 1,3‐dimethyl‐6‐nitro‐5‐arylpyrido[2,3‐d]pyrimidine‐2,4(1H,3H)‐diones starting from 6‐[(dimethylamino)methylene‐amino]‐1,3‐dimethyluracil, aromatic aldehydes and nitromethane using magnetically active CuFe2O4 catalyst system under microwave irradiation and solvent‐less condition. The current methodology is a valued addition to the existing procedures of 5‐arylpyrido[2,3‐d]pyrimidines syntheses making best use of the diene behavior of 6‐[(dimethylamino)methylene‐amino]‐1,3‐dimethyluracil. However, heterogeneous catalysis has been employed for the first time to do the syntheses by carrying out [4 + 2]cycloaddition reaction between 6‐[(dimethylamino)methylene‐amino]‐1,3‐dimethyluracil and in situ generated 2‐(2‐nitrovinyl)arenes/heteroarenes. The methodology is highly time‐economic, and along with other features like easy recovery and good reusability of the catalyst, simple operating procedure, wide substrate scope, and good to excellent product yield, it offers the chemists a reaction protocol worth trying for the syntheses of 1,3‐dimethyl‐6‐nitro‐5‐arylpyrido[2,3‐d]pyrimidine‐2,4(1H,3H)‐diones. While laboratory prepared catalyst system was characterized using FT‐IR, XRD, SEM‐EDX, VSM, XPS and TEM analysis, all the synthesized compounds have been characterized using 1H and 13C NMR spectroscopy and HRMS.
中文翻译:

在微波辐射和无溶剂条件下,可磁回收的铁氧体铜催化1,3-二甲基-6-硝基-5-硝基芳基吡啶[2,3-d]嘧啶-2,4(1H,3H)-二酮的级联合成
近年来,具有磁性的CuFe 2 O 4纳米颗粒在异质催化领域中引起了极大的兴趣,因为它们易于制备并且可以轻松地从反应系统中回收。在这里,我们报告了从6- [ 1]开始级联合成1,3-二甲基-6-硝基-5-芳基吡啶[2,3 - d ]嘧啶-2,4(1 H,3 H)-二酮的工作。 (二甲基氨基)亚甲基氨基] -1,3-二甲基尿嘧啶,芳香醛和硝基甲烷,在微波辐射和无溶剂条件下,使用具有磁性的CuFe 2 O 4催化剂体系。当前的方法是对5-芳基吡啶并[2,3- d]嘧啶的合成可最有效地利用6-[((二甲基氨基)亚甲基-氨基] -1,3-二甲基尿嘧啶的二烯行为。然而,通过在6-[((二甲基氨基)亚甲基-氨基] -1,3-二甲基尿嘧啶和原位生成的2-(2)之间进行[4 + 2]环加成反应,首次采用了非均相催化进行合成-硝基乙烯基)芳烃/杂芳烃。该方法具有很高的时间经济性,并具有催化剂易于回收和良好的可重复使用性,操作步骤简单,底物范围宽,产物收率高至优异等特点,它为化学家提供了值得尝试的反应方案1,3-二甲基-6-硝基-5-芳基吡啶[2,3 - d ]嘧啶-2,4(1 H,3 H)diones。使用FT-IR,XRD,SEM-EDX,VSM,XPS和TEM分析表征实验室制备的催化剂体系时,所有合成的化合物均使用1 H和13 C NMR光谱和HRMS进行了表征。
更新日期:2020-11-10
中文翻译:

在微波辐射和无溶剂条件下,可磁回收的铁氧体铜催化1,3-二甲基-6-硝基-5-硝基芳基吡啶[2,3-d]嘧啶-2,4(1H,3H)-二酮的级联合成
近年来,具有磁性的CuFe 2 O 4纳米颗粒在异质催化领域中引起了极大的兴趣,因为它们易于制备并且可以轻松地从反应系统中回收。在这里,我们报告了从6- [ 1]开始级联合成1,3-二甲基-6-硝基-5-芳基吡啶[2,3 - d ]嘧啶-2,4(1 H,3 H)-二酮的工作。 (二甲基氨基)亚甲基氨基] -1,3-二甲基尿嘧啶,芳香醛和硝基甲烷,在微波辐射和无溶剂条件下,使用具有磁性的CuFe 2 O 4催化剂体系。当前的方法是对5-芳基吡啶并[2,3- d]嘧啶的合成可最有效地利用6-[((二甲基氨基)亚甲基-氨基] -1,3-二甲基尿嘧啶的二烯行为。然而,通过在6-[((二甲基氨基)亚甲基-氨基] -1,3-二甲基尿嘧啶和原位生成的2-(2)之间进行[4 + 2]环加成反应,首次采用了非均相催化进行合成-硝基乙烯基)芳烃/杂芳烃。该方法具有很高的时间经济性,并具有催化剂易于回收和良好的可重复使用性,操作步骤简单,底物范围宽,产物收率高至优异等特点,它为化学家提供了值得尝试的反应方案1,3-二甲基-6-硝基-5-芳基吡啶[2,3 - d ]嘧啶-2,4(1 H,3 H)diones。使用FT-IR,XRD,SEM-EDX,VSM,XPS和TEM分析表征实验室制备的催化剂体系时,所有合成的化合物均使用1 H和13 C NMR光谱和HRMS进行了表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号