当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium Catalyzed C−H Amidation and Carbocyclization using Isocyanates: An Access to Amidated 2‐phenylphthalazine‐1,4‐diones and Indazolo[1,2‐b]phthalazine‐triones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-10 , DOI: 10.1002/adsc.202001146 Pidiyara Karishma 1 , Alisha Gogia 2 , Sanjay K. Mandal 2 , Rajeev Sakhuja 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-10 , DOI: 10.1002/adsc.202001146 Pidiyara Karishma 1 , Alisha Gogia 2 , Sanjay K. Mandal 2 , Rajeev Sakhuja 1
Affiliation
|
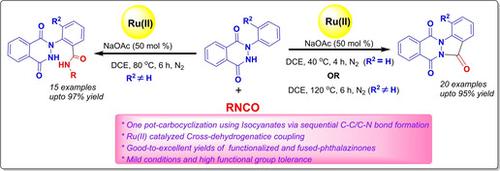
A direct carbocyclization of 2‐aryl‐2,3‐dihydrophthalazine‐1,4‐diones is achieved using isocyanates as carbonyl source via Ru(II)‐catalyzed sequential ortho‐amidation followed by intramolecular nucleophilic substitution, delivering substituted indazolo[1,2‐b]phthalazine‐triones in good‐to‐excellent yields. For ortho‐substituted 2‐aryl‐2,3‐dihydrophthalazine‐1,4‐diones, the corresponding amidated products were also isolated in excellent yields by modifying the reaction parameters. Application of isocyanates as carbonyl source, high functional group tolerance on the two coupling partners and diverse chemical transformation of the synthesized fused and functionalized phthalazinones are the key highlights of the work.
中文翻译:

钌催化的异氰酸酯化CH-H酰胺化和碳环化:酰胺化的2-苯基酞嗪-1,4-二酮和吲唑并[1,2-b]酞嗪三酮的获得
使用异氰酸酯作为羰基来源,通过Ru(II)催化的顺序邻位酰胺化反应,然后进行分子内亲核取代,将取代的吲唑并[ 2,2-]-2-芳基-2,3-二氢酞嗪-1,4-二酮直接碳环化[1,2] ‐ b ]酞嗪三酮,收率良好。对于邻位取代的2-芳基-2,3-二氢邻苯二甲酸1,4-二酮,还可以通过修改反应参数以高收率分离出相应的酰胺化产物。异氰酸酯作为羰基来源的应用,在两个偶联配偶体上的高官能团耐受性以及合成的熔融和官能化邻苯二氮酮的多种化学转化是这项工作的重点。
更新日期:2020-11-10
中文翻译:

钌催化的异氰酸酯化CH-H酰胺化和碳环化:酰胺化的2-苯基酞嗪-1,4-二酮和吲唑并[1,2-b]酞嗪三酮的获得
使用异氰酸酯作为羰基来源,通过Ru(II)催化的顺序邻位酰胺化反应,然后进行分子内亲核取代,将取代的吲唑并[ 2,2-]-2-芳基-2,3-二氢酞嗪-1,4-二酮直接碳环化[1,2] ‐ b ]酞嗪三酮,收率良好。对于邻位取代的2-芳基-2,3-二氢邻苯二甲酸1,4-二酮,还可以通过修改反应参数以高收率分离出相应的酰胺化产物。异氰酸酯作为羰基来源的应用,在两个偶联配偶体上的高官能团耐受性以及合成的熔融和官能化邻苯二氮酮的多种化学转化是这项工作的重点。









































 京公网安备 11010802027423号
京公网安备 11010802027423号