当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold‐Catalyzed Bicyclic and [3+2]‐Annulations of Internal Propargyl Alcohols with Nitrones and Imines To Yield to Two Distinct Heterocycles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-10 , DOI: 10.1002/adsc.202001119 Sayaji Arjun More, Tzu‐Hsuan Chao, Mu‐Jeng Cheng, Rai‐Shung Liu
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-10 , DOI: 10.1002/adsc.202001119 Sayaji Arjun More, Tzu‐Hsuan Chao, Mu‐Jeng Cheng, Rai‐Shung Liu
|
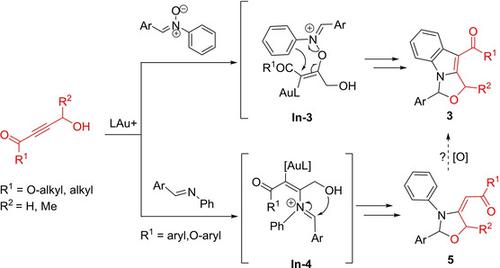
A gold‐catalyzed synthesis of 1,3‐dihydrooxazolo[3,4‐a]indoles from 1‐oxo‐3‐yn‐4‐ols and nitrones is described; this new bicyclic annulation presents the first examples that internal alkynes can react with nitrones to undergo an oxoarylation route. DFT calculations indicate a [3,3]‐sigmatropic shift of initial alkenylgold intermediates to elude the intermediacy of gold carbenes. We also developed new [3+2]‐annulations of the same 1‐oxo‐3‐yn‐4‐ols with imines, yielding oxazolidin‐4‐ylidene derivatives efficiently. The tethered alcohols of these 1‐oxo‐3‐ynes allow trapping of their metastable 2‐azadienium intermediates to enable a novel annulation. Our mechanistic analysis indicates that the two products, despite their structural relevance, are produced from two independent systems.
中文翻译:

含金和亚胺的金催化双环和内部炔丙基醇的[3 + 2]环化产生两个不同的杂环。
描述了一种由金催化的由1-氧代3-yn-4-醇和硝酮合成的1,3-二氢恶唑并[3,4- a ]吲哚的方法;这种新的双环环化反应是第一个实例,表明内部炔烃可以与硝酮反应进行羰基化反应。DFT计算表明,初始烯基金中间体发生了[3,3]-σ位移,从而避开了金卡宾的中间产物。我们还用亚胺开发了相同的1-oxo-3-yn-4醇与新的[3 + 2]环,有效地生成了oxazolidin-4-yylne衍生物。这些1-oxo-3-ynes的束缚醇可捕获其亚稳的2-azadienium中间体,从而实现新颖的环化反应。我们的机械分析表明,尽管两种产品在结构上相关,但它们是由两个独立的系统生产的。
更新日期:2021-01-19
中文翻译:

含金和亚胺的金催化双环和内部炔丙基醇的[3 + 2]环化产生两个不同的杂环。
描述了一种由金催化的由1-氧代3-yn-4-醇和硝酮合成的1,3-二氢恶唑并[3,4- a ]吲哚的方法;这种新的双环环化反应是第一个实例,表明内部炔烃可以与硝酮反应进行羰基化反应。DFT计算表明,初始烯基金中间体发生了[3,3]-σ位移,从而避开了金卡宾的中间产物。我们还用亚胺开发了相同的1-oxo-3-yn-4醇与新的[3 + 2]环,有效地生成了oxazolidin-4-yylne衍生物。这些1-oxo-3-ynes的束缚醇可捕获其亚稳的2-azadienium中间体,从而实现新颖的环化反应。我们的机械分析表明,尽管两种产品在结构上相关,但它们是由两个独立的系统生产的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号