当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coadministration of endothelial and smooth muscle cells derived from human induced pluripotent stem cells as a therapy for critical limb ischemia
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-11-11 , DOI: 10.1002/sctm.20-0132 Jin Ju Park 1 , Yang Woo Kwon 1 , Jeong Won Kim 2 , Gyu Tae Park 1 , Jung Won Yoon 1 , Ye Seul Kim 1 , Da Sol Kim 1 , Sang Mo Kwon 1 , Sun Sik Bae 1 , Kinarm Ko 3 , Chang-Seok Kim 2 , Jae Ho Kim 1, 4
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-11-11 , DOI: 10.1002/sctm.20-0132 Jin Ju Park 1 , Yang Woo Kwon 1 , Jeong Won Kim 2 , Gyu Tae Park 1 , Jung Won Yoon 1 , Ye Seul Kim 1 , Da Sol Kim 1 , Sang Mo Kwon 1 , Sun Sik Bae 1 , Kinarm Ko 3 , Chang-Seok Kim 2 , Jae Ho Kim 1, 4
Affiliation
|
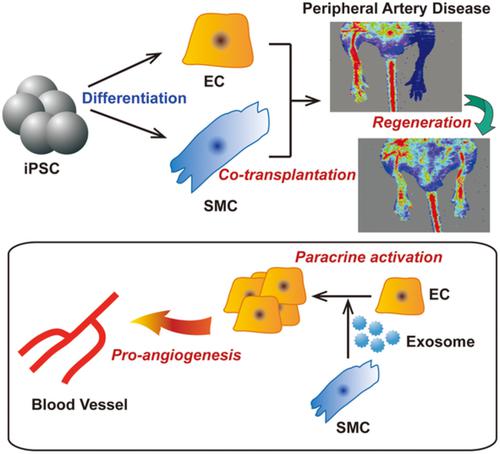
Critical limb ischemia is a condition in which tissue necrosis occurs due to arterial occlusion, resulting in limb amputation in severe cases. Both endothelial cells (ECs) and vascular smooth muscle cells (SMCs) are needed for the regeneration of peripheral arteries in ischemic tissues. However, it is difficult to isolate and cultivate primary EC and SMC from patients for therapeutic angiogenesis. Induced pluripotent stem cells (iPSCs) are regarded as useful stem cells due to their pluripotent differentiation potential. In this study, we explored the therapeutic efficacy of human iPSC‐derived EC and iPSC‐derived SMC in peripheral artery disease model. After the induction of mesodermal differentiation of iPSC, CD34+ progenitor cells were isolated by magnetic‐activated cell sorting. Cultivation of the CD34+ progenitor cells in endothelial culture medium induced the expression of endothelial markers and phenotypes. Moreover, the CD34+ cells could be differentiated into SMC by cultivation in SMC culture medium. In a murine hindlimb ischemia model, cotransplantation of EC with SMC improved blood perfusion and increased the limb salvage rate in ischemic limbs compared to transplantation of either EC or SMC alone. Moreover, cotransplantation of EC and SMC stimulated angiogenesis and led to the formation of capillaries and arteries/arterioles in vivo. Conditioned medium derived from SMC stimulated the migration, proliferation, and tubulation of EC in vitro, and these effects were recapitulated by exosomes isolated from the SMC‐conditioned medium. Together, these results suggest that iPSC‐derived SMC enhance the therapeutic efficacy of iPSC‐derived EC in peripheral artery disease via an exosome‐mediated paracrine mechanism.
中文翻译:

来自人诱导多能干细胞的内皮细胞和平滑肌细胞的共同给药作为治疗严重肢体缺血的方法
严重的肢体缺血是由于动脉闭塞而发生组织坏死,严重时导致肢体截肢的病症。缺血组织中外周动脉的再生需要内皮细胞 (EC) 和血管平滑肌细胞 (SMC)。然而,很难从患者中分离和培养原发性 EC 和 SMC 用于治疗性血管生成。诱导多能干细胞 (iPSC) 因其多能分化潜能而被认为是有用的干细胞。在本研究中,我们探讨了人 iPSC 衍生的 EC 和 iPSC 衍生的 SMC 在外周动脉疾病模型中的治疗效果。诱导 iPSC 中胚层分化后,通过磁激活细胞分选分离CD34 +祖细胞。CD34的培养+内皮培养基中的祖细胞诱导内皮标志物和表型的表达。此外,CD34 +通过在SMC培养基中培养,细胞可以分化为SMC。在小鼠后肢缺血模型中,与单独移植 EC 或 SMC 相比,EC 与 SMC 的共同移植改善了血液灌注并增加了缺血肢体的保肢率。此外,EC 和 SMC 的共同移植刺激血管生成并导致体内毛细血管和动脉/小动脉的形成。源自 SMC 的条件培养基在体外刺激了 EC 的迁移、增殖和管状形成,这些作用被从 SMC 条件培养基中分离出来的外泌体所概括。总之,这些结果表明,iPSC 衍生的 SMC 通过外泌体介导的旁分泌机制增强了 iPSC 衍生的 EC 在外周动脉疾病中的治疗效果。
更新日期:2020-11-11
中文翻译:

来自人诱导多能干细胞的内皮细胞和平滑肌细胞的共同给药作为治疗严重肢体缺血的方法
严重的肢体缺血是由于动脉闭塞而发生组织坏死,严重时导致肢体截肢的病症。缺血组织中外周动脉的再生需要内皮细胞 (EC) 和血管平滑肌细胞 (SMC)。然而,很难从患者中分离和培养原发性 EC 和 SMC 用于治疗性血管生成。诱导多能干细胞 (iPSC) 因其多能分化潜能而被认为是有用的干细胞。在本研究中,我们探讨了人 iPSC 衍生的 EC 和 iPSC 衍生的 SMC 在外周动脉疾病模型中的治疗效果。诱导 iPSC 中胚层分化后,通过磁激活细胞分选分离CD34 +祖细胞。CD34的培养+内皮培养基中的祖细胞诱导内皮标志物和表型的表达。此外,CD34 +通过在SMC培养基中培养,细胞可以分化为SMC。在小鼠后肢缺血模型中,与单独移植 EC 或 SMC 相比,EC 与 SMC 的共同移植改善了血液灌注并增加了缺血肢体的保肢率。此外,EC 和 SMC 的共同移植刺激血管生成并导致体内毛细血管和动脉/小动脉的形成。源自 SMC 的条件培养基在体外刺激了 EC 的迁移、增殖和管状形成,这些作用被从 SMC 条件培养基中分离出来的外泌体所概括。总之,这些结果表明,iPSC 衍生的 SMC 通过外泌体介导的旁分泌机制增强了 iPSC 衍生的 EC 在外周动脉疾病中的治疗效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号