当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The soluble cytoplasmic N‐terminal domain of the FocA channel gates bidirectional formate translocation
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-10 , DOI: 10.1111/mmi.14641 Michelle Kammel 1 , Doreen Hunger 1 , Robert Gary Sawers 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-10 , DOI: 10.1111/mmi.14641 Michelle Kammel 1 , Doreen Hunger 1 , Robert Gary Sawers 1
Affiliation
|
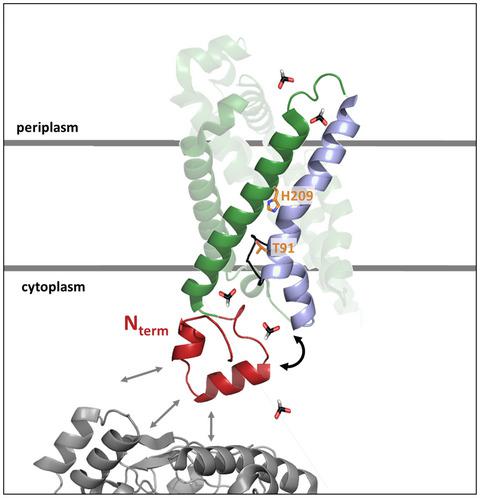
FocA belongs to the pentameric FNT (formate‐nitrite transporter) superfamily of anion channels, translocating formate bidirectionally across the cytoplasmic membrane of Escherichia coli and other microorganisms. While the membrane‐integral core of FocA shares considerable amino acid sequence conservation with other FNT family members, the soluble cytoplasmic N‐terminal domain does not. To analyze the potential biochemical function of FocA’s N‐terminal domain in vivo, we constructed truncation derivatives and amino acid‐exchange variants, and determined their ability to translocate formate across the membrane of E. coli cells by monitoring intracellular formate levels using a formate‐sensitive reporter system. Analysis of strains synthesizing these FocA variants provided insights into formate efflux. Strains lacking the ability to generate formate intracellularly allowed us to determine whether these variants could import formate or its toxic chemical analog hypophosphite. Our findings reveal that the N‐terminal domain of FocA is crucial for bidirectional FocA‐dependent permeation of formate across the membrane. Moreover, we show that an amino acid sequence motif and secondary structural features of the flexible N‐terminal domain are important for formate translocation, and efflux/influx is influenced by pyruvate formate‐lyase. The soluble N‐terminal domain is, therefore, essential for bidirectional formate translocation by FocA, suggesting a “gate‐keeper” function controlling anion accessibility.
中文翻译:

FocA 通道的可溶性细胞质 N 端结构域控制双向甲酸易位
福卡属于五聚体FNT(˚F ormate- Ñ itrite吨ransporter)超家族的阴离子通道,跨越胞质膜易位甲酸双向大肠杆菌和其他微生物。虽然 FocA 的膜整合核心与其他 FNT 家族成员共享相当多的氨基酸序列保守性,但可溶性细胞质 N 端结构域却没有。为了分析 FocA 的 N 端结构域在体内的潜在生化功能,我们构建了截断衍生物和氨基酸交换变体,并确定了它们跨大肠杆菌膜转运甲酸的能力通过使用甲酸敏感报告系统监测细胞内甲酸水平。对合成这些 FocA 变体的菌株的分析提供了对甲酸流出的见解。缺乏在细胞内产生甲酸的能力的菌株使我们能够确定这些变体是否可以输入甲酸或其有毒化学类似物次磷酸盐。我们的研究结果表明,FocA 的 N 端结构域对于甲酸盐双向 FocA 依赖性渗透至关重要。此外,我们表明灵活的 N 端结构域的氨基酸序列基序和二级结构特征对甲酸易位很重要,外排/流入受丙酮酸甲酸裂解酶的影响。因此,可溶性 N 端结构域对于 FocA 的双向甲酸易位至关重要,
更新日期:2020-11-10
中文翻译:

FocA 通道的可溶性细胞质 N 端结构域控制双向甲酸易位
福卡属于五聚体FNT(˚F ormate- Ñ itrite吨ransporter)超家族的阴离子通道,跨越胞质膜易位甲酸双向大肠杆菌和其他微生物。虽然 FocA 的膜整合核心与其他 FNT 家族成员共享相当多的氨基酸序列保守性,但可溶性细胞质 N 端结构域却没有。为了分析 FocA 的 N 端结构域在体内的潜在生化功能,我们构建了截断衍生物和氨基酸交换变体,并确定了它们跨大肠杆菌膜转运甲酸的能力通过使用甲酸敏感报告系统监测细胞内甲酸水平。对合成这些 FocA 变体的菌株的分析提供了对甲酸流出的见解。缺乏在细胞内产生甲酸的能力的菌株使我们能够确定这些变体是否可以输入甲酸或其有毒化学类似物次磷酸盐。我们的研究结果表明,FocA 的 N 端结构域对于甲酸盐双向 FocA 依赖性渗透至关重要。此外,我们表明灵活的 N 端结构域的氨基酸序列基序和二级结构特征对甲酸易位很重要,外排/流入受丙酮酸甲酸裂解酶的影响。因此,可溶性 N 端结构域对于 FocA 的双向甲酸易位至关重要,









































 京公网安备 11010802027423号
京公网安备 11010802027423号