Solid State Ionics ( IF 3.0 ) Pub Date : 2020-11-11 , DOI: 10.1016/j.ssi.2020.115506 Yayu Guo , Huilan Guan , Wenxiu Peng , Xuelei Li , Yue Ma , Dawei Song , Hongzhou Zhang , Chunliang Li , Lianqi Zhang
|
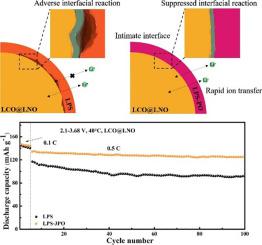
Li7P3S11 (70Li2S·30P2S5) sulfide is a promising electrolyte candidate for all-solid-state lithium batteries (ASSLBs) owing to its high ionic conducivity and wide electrochemical stability window. However, the sensitivity to moisture and the poor interfacial compatibility with electrodes limit the conventional application of Li7P3S11. Herein, we present a substitution strategy to produce a O-incorporated 70Li2S·(30-x)P2S5·xP2O5 (mol%) sulfide electrolyte to address above issues. The elaborately designed electrolyte is prepared via wet vibration ball milling technique with P2O5 substitution content of 1, 3 and 5. As a result, the 70Li2S·27P2S5·3P2O5 electrolyte displays the highest ionic conductivity of 2.61× 10−3 S cm−1 which outperforms than that of pristine Li7P3S11 (ionic conductivity of 1.35 × 10−3 S cm−1) and the assembled LiCoO2@LiNbO3/70Li2S·27P2S5·3P2O5/Li-In ASSLB exhibits the superior capacity retention of 93.2% after 100 cycles at 0.5C. The reason for the developed electrochemical performance lies in the substitution of the non-bridging sulfur atom with bridging oxygen atom, from which the interaction between electrolyte and lithium ions is weakened, thereby the rapid transfer of lithium ions is facilitated. Furthermore, Raman and XRD results indicate that the adverse interfacial reactions are suppressed through P2O5 substitution. This work suggests that P2O5 substitution is an effective strategy for sulfide electrolyte toward high energy all-solid-state lithium batteries.
中文翻译:

通过P 2 O 5替代全固态锂电池增强Li 7 P 3 S 11电解质的电化学性能
Li 7 P 3 S 11(70Li 2 S·30P 2 S 5)硫化物由于具有高离子电导率和宽的电化学稳定性范围,因此是用于全固态锂电池(ASSLB)的有希望的电解质候选者。然而,对湿气的敏感性和与电极的差的界面相容性限制了Li 7 P 3 S 11的常规应用。在这里,我们提出一种替代策略,以生产掺入O的70Li 2 S·(30-x)P 2 S 5 ·xP 2 O 5(mol%)硫化物电解质来解决上述问题。通过湿振动球磨技术制备精心设计的电解质,P 2 O 5的取代含量分别为1、3和5。结果,70Li 2 S·27P 2 S 5 ·3P 2 O 5电解质显示出最高的离子电导率比原始Li 7 P 3 S 11(离子电导率为1.35×10 -3 S cm -1)和组装的LiCoO 2 @LiNbO 3 / 70Li的性能更好的2.61×10 -3 S cm -12 S·27P 2 S 5 ·3P 2 O 5 / Li-In ASSLB在0.5C下循环100次后,显示出93.2%的优异容量保持率。电化学性能提高的原因在于,用桥连的氧原子取代了非桥连的硫原子,由此削弱了电解质与锂离子之间的相互作用,从而促进了锂离子的快速转移。此外,拉曼和XRD结果表明不良的界面反应通过P 2 O 5取代得到抑制。这项工作表明P 2 O 5 替代是将硫化物电解质用于高能全固态锂电池的有效策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号