Science Bulletin ( IF 18.8 ) Pub Date : 2020-11-09 , DOI: 10.1016/j.scib.2020.11.003 Huifang Xian 1 , Wanming Huang 2 , Tingzhe Sun 3 , Shuai Yang 4 , Chuanxia Zhang 4 , Jun Wang 2 , Yuxia Zhang 2 , Jun Cui 4
|
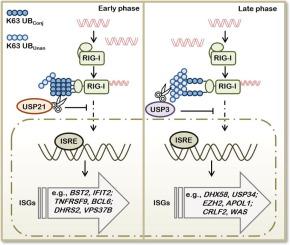
Ubiquitination plays a crucial role in retinoic acid-inducible gene I (RIG-I)-induced antiviral responses. However, the precise regulatory mechanisms of RIG-I activity mediated by conjugated and unanchored ubiquitin chains remain to be determined. In this study, we discovered that T55 of RIG-I was required for its binding ability for the unanchored ubiquitin chains. Experimental and mathematical analysis showed that unanchored ubiquitin chains associated with RIG-I were essential for sustained activation of type I interferon (IFN) signaling. Transcriptomics study revealed that the binding of RIG-I with unanchored ubiquitin chains additionally regulated the expression of a subset of metabolic and cell fate decision genes. Moreover, we found that ubiquitin-specific peptidase 21 (USP21) and USP3 deubiquitinate conjugated and unanchored ubiquitin chains on RIG-I respectively. Taken together, characterization of the regulation mode and functions of conjugated ubiquitination and the unconjugated ubiquitin chain-binding of RIG-I may provide means to fine-tune RIG-I-mediated type I IFN signaling.
中文翻译:

未锚定的泛素链维持 RIG-I 诱导的干扰素-I 激活并控制选择性基因表达
泛素化在视黄酸诱导基因 I (RIG-I) 诱导的抗病毒反应中起着至关重要的作用。然而,由共轭和未锚定的泛素链介导的 RIG-I 活性的精确调节机制仍有待确定。在这项研究中,我们发现 RIG-I 的 T55 是其对未锚定泛素链的结合能力所必需的。实验和数学分析表明,与 RIG-I 相关的未锚定泛素链对于持续激活 I 型干扰素 (IFN) 信号传导至关重要。转录组学研究表明,RIG-I 与未锚定泛素链的结合额外调节了一部分代谢和细胞命运决定基因的表达。而且,我们发现泛素特异性肽酶 21 (USP21) 和 USP3 分别去泛素化 RIG-I 上的共轭和非锚定泛素链。总之,共轭泛素化的调节模式和功能的表征以及 RIG-I 的未共轭泛素链结合可能提供微调 RIG-I 介导的 I 型 IFN 信号传导的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号