Life Sciences ( IF 5.2 ) Pub Date : 2020-11-11 , DOI: 10.1016/j.lfs.2020.118740 Samirul Bashir , Mariam Banday , Ozaira Qadri , Arif Bashir , Nazia Hilal , Nida-i-Fatima , Stephen Rader , Khalid Majid Fazili
|
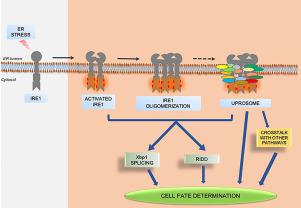
The endoplasmic reticulum is primarily responsible for protein folding and maturation. However, the organelle is subject to varied stress conditions from time to time, which lead to the activation of a signaling program known as the Unfolded Protein Response (UPR) pathway. This pathway, upon sensing any disturbance in the protein-folding milieu sends signals to the nucleus and cytoplasm in order to restore homeostasis. One of the prime UPR signaling sensors is Inositol-requiring enzyme 1 (IRE1); an ER membrane embedded protein with dual enzyme activities, kinase and endoribonuclease. The ribonuclease activity of IRE1 results in Xbp1 splicing in mammals or Hac1 splicing in yeast. However, IRE1 can switch its substrate specificity to the mRNAs that are co-transnationally transported to the ER, a phenomenon known as Regulated IRE1 Dependent Decay (RIDD). IRE1 is also reported to act as a principal molecule that coordinates with other proteins and signaling pathways, which in turn might be responsible for its regulation. The current review highlights studies on IRE1 explaining the structural features and molecular mechanism behind its ribonuclease outputs. The emphasis is also laid on the molecular effectors, which directly or indirectly interact with IRE1 to either modulate its function or connect it to other pathways. This is important in understanding the functional pleiotropy of IRE1, by which it can switch its activity from pro-survival to pro-apoptotic, thus determining the fate of cells.
中文翻译:

UPR信号传感器IRE1的分子机制和功能多样性
内质网主要负责蛋白质折叠和成熟。但是,细胞器会不时受到变化的压力条件的影响,这会导致信号程序的激活,该程序被称为未折叠蛋白反应(UPR)途径。在检测到蛋白质折叠环境中的任何干扰后,该途径会将信号发送至细胞核和细胞质,以恢复体内平衡。UPR信号转导的主要传感器之一是需要肌醇的酶1(IRE1)。具有双重酶活性,激酶和核糖核酸内切酶的内质网膜嵌入蛋白。IRE1的核糖核酸酶活性导致在哺乳动物中剪接Xbp1或在酵母中剪接Hac1。但是,IRE1可以将其底物特异性切换为协同跨国转运至ER的mRNA,一种称为IRE1依赖衰减受控(RIDD)的现象。IRE1也据报道是与其他蛋白质和信号传导途径协调的主要分子,这可能又对其调节负责。本综述着重介绍了有关IRE1的研究,解释了其核糖核酸酶输出背后的结构特征和分子机理。重点还放在分子效应子上,其直接或间接与IRE1相互作用以调节其功能或将其连接至其他途径。这对于了解IRE1的功能多效性很重要,通过它它可以将IRE1的活性从存活前的状态转换为凋亡前的状态,从而确定细胞的命运。反过来可能对其监管负责。本综述着重介绍了有关IRE1的研究,解释了其核糖核酸酶输出背后的结构特征和分子机理。重点还放在分子效应子上,其直接或间接与IRE1相互作用以调节其功能或将其连接至其他途径。这对于了解IRE1的功能多效性很重要,通过它它可以将IRE1的活性从存活前的状态转换为凋亡前的状态,从而确定细胞的命运。反过来可能对其监管负责。本综述着重介绍了有关IRE1的研究,解释了其核糖核酸酶输出背后的结构特征和分子机理。重点还放在分子效应子上,其直接或间接与IRE1相互作用以调节其功能或将其连接至其他途径。这对于了解IRE1的功能多效性很重要,通过它它可以将IRE1的活性从存活前的状态转换为凋亡前的状态,从而确定细胞的命运。直接或间接与IRE1相互作用以调节其功能或将其连接至其他途径。这对于了解IRE1的功能多效性很重要,通过它它可以将IRE1的活性从存活前的状态转换为凋亡前的状态,从而确定细胞的命运。直接或间接与IRE1相互作用以调节其功能或将其连接至其他途径。这对于了解IRE1的功能多效性很重要,通过它它可以将IRE1的活性从存活前的状态转换为凋亡前的状态,从而确定细胞的命运。











































 京公网安备 11010802027423号
京公网安备 11010802027423号