Journal of Bioscience and Bioengineering ( IF 2.3 ) Pub Date : 2020-11-11 , DOI: 10.1016/j.jbiosc.2020.10.010 Saša Vatić , Nemanja Mirković , Jelica R. Milošević , Branko Jovčić , Natalija Đ. Polović
|
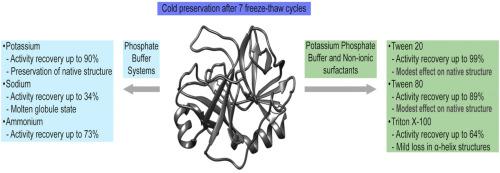
Trypsin is a serine protease with important applications such as protein sequencing and tissue dissociation. Preserving protein structure and its activity during freeze-thawing and prolonging its shelf life is one of the most interesting tasks in biochemistry. In the present study, trypsin cryoprotection was achieved by altering buffer composition. Sodium phosphate buffer at pH 8.0 led to pH shift-induced destabilization of trypsin and formation of a molten globule, followed by significant activity loss (about 70%). Potassium phosphate and ammonium bicarbonate buffers at pH 8.0 were used with up to 90% activity recovery rate after 7 freeze-thaw cycles. The addition of non-ionic surfactants Tween 20 and Tween 80 led to up to 99% activity recovery rate. Amide I region changes, corresponding to specific secondary structures in the Fourier transform infrared (FTIR) spectrum, were modest in the case of Tween 20 and Tween 80. On the other hand, the addition of Triton X-100 led to the destabilization of α-helicoidal segments of trypsin structure after 7 freeze-thaw cycles but also increased protein substrate availability.
中文翻译:

在离子和非离子表面活性剂存在下的胰蛋白酶活性和冻融稳定性
胰蛋白酶是一种丝氨酸蛋白酶,具有重要的应用,例如蛋白质测序和组织解离。在冻融过程中保持蛋白质结构及其活性并延长其保质期是生物化学中最有趣的任务之一。在本研究中,胰蛋白酶的冷冻保护是通过改变缓冲液的组成来实现的。pH 8.0的磷酸钠缓冲液导致pH偏移引起的胰蛋白酶不稳定并形成熔融小球,随后活性明显下降(约70%)。在7个冻融循环后,使用pH 8.0的磷酸钾和碳酸氢铵缓冲液,回收率高达90%。加入非离子型表面活性剂Tween 20和Tween 80可使活性恢复率提高到99%。酰胺一区发生变化,











































 京公网安备 11010802027423号
京公网安备 11010802027423号