当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron Carbonyl Complexes Containing N,C,S-Tridentate Ligands with Quinoline, Vinyl, and Benzenethiolate Units
Organometallics ( IF 2.5 ) Pub Date : 2020-11-06 , DOI: 10.1021/acs.organomet.0c00621 Yuta Masuda 1 , Yuki Yagami 1 , Kotomi Nakazawa 1 , Masakazu Hirotsu 1
Organometallics ( IF 2.5 ) Pub Date : 2020-11-06 , DOI: 10.1021/acs.organomet.0c00621 Yuta Masuda 1 , Yuki Yagami 1 , Kotomi Nakazawa 1 , Masakazu Hirotsu 1
Affiliation
|
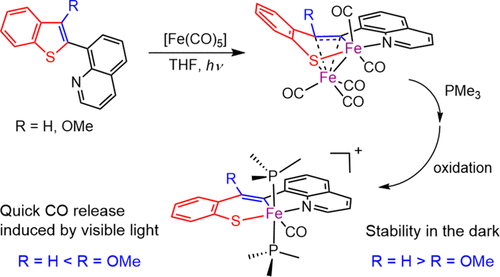
Iron(III) carbonyl complexes of N,C,S-tridentate ligands containing quinoline-N, vinyl-C, and benzenethiolate-S donors were synthesized by three steps from quinolyl-substituted benzothiophenes via C–S bond cleavage, and their photoreactivity was investigated. The starting complexes [Fe2(μ-L)(CO)5] (L = qvbt and qvbt-OMe) were synthesized by the photoreactions of the ligand precursors with [Fe(CO)5], where qvbt and qvbt-OMe are a doubly deprotonated form of 2-[2-(quinolin-8-yl)vinyl]benzenethiol and its methoxy derivative, respectively. The methoxy group is bound to the vinyl moiety of the ligand, which is the β-position to Fe in the C,S-chelate. The N,C,S-pincer iron(II) carbonyl complexes trans-[Fe(L)(CO)(PMe3)2] were obtained by treatment of [Fe2(μ-L)(CO)5] with PMe3. One-electron oxidation of the iron(II) complexes afforded the N,C,S-pincer iron(III) carbonyl complexes trans-[Fe(L)(CO)(PMe3)2]PF6. The iron(III) carbonyl complex of qvbt was adequately stable in both the solid and solution state, whereas the qvbt-OMe complex underwent thermal decomposition in solution. The instability is related to the steric demand of the methoxy group in the coordinated ligand. Both iron(III) complexes showed the CO-releasing properties induced by visible light in dichloromethane, which is enhanced by the methoxy group on the vinyl moiety.
中文翻译:

含N,C,S三叉配体的喹啉,乙烯基和苯硫醇酯单元的铁羰基配合物
由喹啉基取代的苯并噻吩经C–S键裂解通过三个步骤合成了N,C,S三齿配体的N,C,S-三齿配体的羰基铁(III)配合物,其光反应性为调查。通过配体前体与[Fe(CO)5 ]的光反应合成了起始配合物[Fe 2(μ-L)(CO)5 ](L = qvbt和qvbt-OMe),其中qvbt和qvbt-OMe为分别是2- [2-(喹啉-8-乙烯基)乙烯基]苯硫醇的双去质子化形式及其甲氧基衍生物。甲氧基与配体的乙烯基部分结合,该乙烯基部分是C,S-螯合物中Fe的β位。N,C,S-cer铁(II)羰基配合物反式-[Fe(L)(CO)(PMe 3))2 ]通过用PMe 3处理[Fe 2(μ-L)(CO)5 ]而获得。铁(II)配合物的单电子氧化提供了N,C,S-钳制铁(III)羰基配合物反式-[Fe(L)(CO)(PMe 3)2 ] PF 6。qvbt的羰基铁(III)络合物在固态和溶液状态下都足够稳定,而qvbt-OMe络合物在溶液中经历热分解。不稳定性与配位体中甲氧基的空间需求有关。两种铁(III)配合物均显示出由二氯甲烷中的可见光诱导的CO释放特性,而乙烯基部分上的甲氧基则增强了该特性。
更新日期:2020-11-23
中文翻译:

含N,C,S三叉配体的喹啉,乙烯基和苯硫醇酯单元的铁羰基配合物
由喹啉基取代的苯并噻吩经C–S键裂解通过三个步骤合成了N,C,S三齿配体的N,C,S-三齿配体的羰基铁(III)配合物,其光反应性为调查。通过配体前体与[Fe(CO)5 ]的光反应合成了起始配合物[Fe 2(μ-L)(CO)5 ](L = qvbt和qvbt-OMe),其中qvbt和qvbt-OMe为分别是2- [2-(喹啉-8-乙烯基)乙烯基]苯硫醇的双去质子化形式及其甲氧基衍生物。甲氧基与配体的乙烯基部分结合,该乙烯基部分是C,S-螯合物中Fe的β位。N,C,S-cer铁(II)羰基配合物反式-[Fe(L)(CO)(PMe 3))2 ]通过用PMe 3处理[Fe 2(μ-L)(CO)5 ]而获得。铁(II)配合物的单电子氧化提供了N,C,S-钳制铁(III)羰基配合物反式-[Fe(L)(CO)(PMe 3)2 ] PF 6。qvbt的羰基铁(III)络合物在固态和溶液状态下都足够稳定,而qvbt-OMe络合物在溶液中经历热分解。不稳定性与配位体中甲氧基的空间需求有关。两种铁(III)配合物均显示出由二氯甲烷中的可见光诱导的CO释放特性,而乙烯基部分上的甲氧基则增强了该特性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号