当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clickable Transformation of Nitriles (RCN) to Oxazolyl Sulfonyl Fluoride Warheads
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.orglett.0c03298 Wan-Yin Fang 1 , Shi-Meng Wang 2 , Zai-Wei Zhang 1 , Hua-Li Qin 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.orglett.0c03298 Wan-Yin Fang 1 , Shi-Meng Wang 2 , Zai-Wei Zhang 1 , Hua-Li Qin 1
Affiliation
|
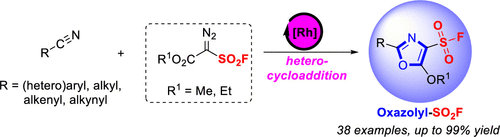
The protocol for simple, efficient, and mild synthesis of oxazolyl sulfonyl fluorides was developed through Rh2(OAc)4-catalyzed annulation of methyl-2-diazo-2-(fluorosulfonyl)acetate (MDF) or its ethyl ester derivative with nitriles. This practical method provides a general and direct route to a unique class of highly functionalized oxazolyl-decorated sulfonyl fluoride warheads with great potential in medicinal chemistry, chemical biology, and drug discovery.
中文翻译:

腈(RCN)向恶唑基磺酰氟战斗部的可点击转化
通过Rh 2(OAc)4催化甲基-2-重氮-2-(氟磺酰基)乙酸甲酯(MDF)或其乙酯衍生物与腈的环化反应,开发了简单,高效,温和地合成恶唑基磺酰氟的方案。这种实用的方法提供了一条通用而直接的途径,可用于生产一类独特的高度功能化的恶唑基修饰的磺酰氟战斗部,在药物化学,化学生物学和药物开发中具有巨大的潜力。
更新日期:2020-11-21
中文翻译:

腈(RCN)向恶唑基磺酰氟战斗部的可点击转化
通过Rh 2(OAc)4催化甲基-2-重氮-2-(氟磺酰基)乙酸甲酯(MDF)或其乙酯衍生物与腈的环化反应,开发了简单,高效,温和地合成恶唑基磺酰氟的方案。这种实用的方法提供了一条通用而直接的途径,可用于生产一类独特的高度功能化的恶唑基修饰的磺酰氟战斗部,在药物化学,化学生物学和药物开发中具有巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号