当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Initial Leuprolide Acetate Release from Poly(d,l-lactide-co-glycolide) in Situ Forming Implants as Studied by Ultraviolet–Visible Imaging
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.molpharmaceut.0c00625 Zhuoxuan Li 1 , Huiling Mu 1 , Susan Weng Larsen 1 , Henrik Jensen 1 , Jesper Østergaard 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-09 , DOI: 10.1021/acs.molpharmaceut.0c00625 Zhuoxuan Li 1 , Huiling Mu 1 , Susan Weng Larsen 1 , Henrik Jensen 1 , Jesper Østergaard 1
Affiliation
|
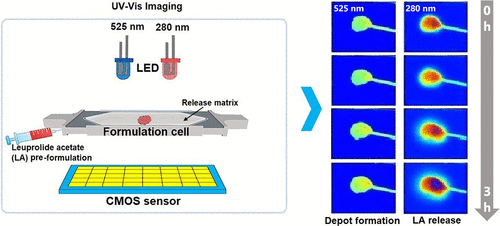
The initial drug release from in situ forming implants is affected by factors such as the physicochemical properties of the active pharmaceutical ingredient, the type of the excipients utilized, and the surrounding environment. The feasibility of UV–vis imaging for characterization of the initial behavior of poly(d,l-lactide-co-glycolide) (PLGA)/1-methyl-2-pyrrolidinone (NMP) in situ forming implants was investigated. The in vitro release of leuprolide acetate (LA) and implant formation in real time were monitored using dual-wavelength imaging at 280 and 525 nm, respectively, in matrices based on agarose gel and hyaluronic acid (HA) solution emulating the subcutaneous matrix. Three hours upon injection of the pre-formulation, approximately 15% of the total amount of LA administered was found in the agarose gel, while 5% was released from the implant into the HA solution. Concurrently, more extensive swelling of the implants in the HA solution as compared to implants in the agarose gel was observed. Transport of both LA and the solvent NMP was investigated using UV–vis imaging in a small-scale cell where the geometry of the formulation was controlled, showing a linear correlation between drug release and solvent escape. Light microscopy showed that the microstructures of the resulting implants in agarose gel and HA solution were different, which may be attributed to the different solvent exchange rates. UV imaging was also used to examine the interaction of LA with the release medium by characterizing the diffusion of LA in agarose gel, HA solution, and phosphate buffered saline. The reduced LA diffusivity in HA solution as compared to agarose gel and the LA distribution coefficient in the agarose gel–HA system indicated the presence of interactions between LA and HA. Our findings show that the external environment affects the solvent exchange kinetics for in situ forming implants in vitro, resulting in different types of initial release behavior. UV–vis imaging in combination with biorelevant matrices may offer an interesting approach in the development of in situ forming implant delivery systems.
中文翻译:

聚(d,l-丙交酯-共-乙交酯)原位成形植入物的醋酸亮丙瑞林初始释放,通过紫外可见成像研究
原位成形植入物的初始药物释放受多种因素的影响,例如活性药物成分的理化性质、所用赋形剂的类型以及周围环境。研究了紫外-可见成像用于表征聚(d,l-丙交酯-共-乙交酯)(PLGA)/1-甲基-2-吡咯烷酮(NMP)原位形成植入物的初始行为的可行性。在体外在基于琼脂糖凝胶和透明质酸 (HA) 溶液模拟皮下基质的基质中,分别使用 280 和 525 nm 的双波长成像实时监测醋酸亮丙瑞林 (LA) 的释放和植入物的形成。注射预制剂三小时后,在琼脂糖凝胶中发现所施用的 LA 总量的约 15%,而 5% 从植入物释放到 HA 溶液中。同时,观察到与在琼脂糖凝胶中的植入物相比,在 HA 溶液中的植入物膨胀更广泛。LA 和溶剂 NMP 的传输在小规模细胞中使用 UV-vis 成像进行研究,其中配方的几何形状受到控制,显示药物释放和溶剂逸出之间的线性相关性。光学显微镜显示,所得植入物在琼脂糖凝胶和 HA 溶液中的微观结构不同,这可能归因于不同的溶剂交换率。UV 成像还用于通过表征 LA 在琼脂糖凝胶、HA 溶液和磷酸盐缓冲盐水中的扩散来检查 LA 与释放介质的相互作用。与琼脂糖凝胶相比,HA 溶液中 LA 的扩散率降低,而琼脂糖凝胶-HA 系统中的 LA 分配系数表明 LA 和 HA 之间存在相互作用。我们的研究结果表明,外部环境影响溶剂交换动力学 UV 成像还用于通过表征 LA 在琼脂糖凝胶、HA 溶液和磷酸盐缓冲盐水中的扩散来检查 LA 与释放介质的相互作用。与琼脂糖凝胶相比,HA 溶液中 LA 的扩散率降低,而琼脂糖凝胶-HA 系统中的 LA 分配系数表明 LA 和 HA 之间存在相互作用。我们的研究结果表明,外部环境影响溶剂交换动力学 UV 成像还用于通过表征 LA 在琼脂糖凝胶、HA 溶液和磷酸盐缓冲盐水中的扩散来检查 LA 与释放介质的相互作用。与琼脂糖凝胶相比,HA 溶液中 LA 的扩散率降低,而琼脂糖凝胶-HA 系统中的 LA 分配系数表明 LA 和 HA 之间存在相互作用。我们的研究结果表明,外部环境影响溶剂交换动力学在体外原位形成植入物,导致不同类型的初始释放行为。UV-vis 成像结合生物相关基质可能为原位成形植入物输送系统的开发提供一种有趣的方法。
更新日期:2020-12-07
中文翻译:

聚(d,l-丙交酯-共-乙交酯)原位成形植入物的醋酸亮丙瑞林初始释放,通过紫外可见成像研究
原位成形植入物的初始药物释放受多种因素的影响,例如活性药物成分的理化性质、所用赋形剂的类型以及周围环境。研究了紫外-可见成像用于表征聚(d,l-丙交酯-共-乙交酯)(PLGA)/1-甲基-2-吡咯烷酮(NMP)原位形成植入物的初始行为的可行性。在体外在基于琼脂糖凝胶和透明质酸 (HA) 溶液模拟皮下基质的基质中,分别使用 280 和 525 nm 的双波长成像实时监测醋酸亮丙瑞林 (LA) 的释放和植入物的形成。注射预制剂三小时后,在琼脂糖凝胶中发现所施用的 LA 总量的约 15%,而 5% 从植入物释放到 HA 溶液中。同时,观察到与在琼脂糖凝胶中的植入物相比,在 HA 溶液中的植入物膨胀更广泛。LA 和溶剂 NMP 的传输在小规模细胞中使用 UV-vis 成像进行研究,其中配方的几何形状受到控制,显示药物释放和溶剂逸出之间的线性相关性。光学显微镜显示,所得植入物在琼脂糖凝胶和 HA 溶液中的微观结构不同,这可能归因于不同的溶剂交换率。UV 成像还用于通过表征 LA 在琼脂糖凝胶、HA 溶液和磷酸盐缓冲盐水中的扩散来检查 LA 与释放介质的相互作用。与琼脂糖凝胶相比,HA 溶液中 LA 的扩散率降低,而琼脂糖凝胶-HA 系统中的 LA 分配系数表明 LA 和 HA 之间存在相互作用。我们的研究结果表明,外部环境影响溶剂交换动力学 UV 成像还用于通过表征 LA 在琼脂糖凝胶、HA 溶液和磷酸盐缓冲盐水中的扩散来检查 LA 与释放介质的相互作用。与琼脂糖凝胶相比,HA 溶液中 LA 的扩散率降低,而琼脂糖凝胶-HA 系统中的 LA 分配系数表明 LA 和 HA 之间存在相互作用。我们的研究结果表明,外部环境影响溶剂交换动力学 UV 成像还用于通过表征 LA 在琼脂糖凝胶、HA 溶液和磷酸盐缓冲盐水中的扩散来检查 LA 与释放介质的相互作用。与琼脂糖凝胶相比,HA 溶液中 LA 的扩散率降低,而琼脂糖凝胶-HA 系统中的 LA 分配系数表明 LA 和 HA 之间存在相互作用。我们的研究结果表明,外部环境影响溶剂交换动力学在体外原位形成植入物,导致不同类型的初始释放行为。UV-vis 成像结合生物相关基质可能为原位成形植入物输送系统的开发提供一种有趣的方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号