当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of CO2 in Sodium Silicate Melts
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-11-09 , DOI: 10.1021/acsearthspacechem.0c00223 Nathan S. Jacobson 1 , Bruce Fegley 2 , Amy C. McAdam 3 , Christine A. Knudson 4
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-11-09 , DOI: 10.1021/acsearthspacechem.0c00223 Nathan S. Jacobson 1 , Bruce Fegley 2 , Amy C. McAdam 3 , Christine A. Knudson 4
Affiliation
|
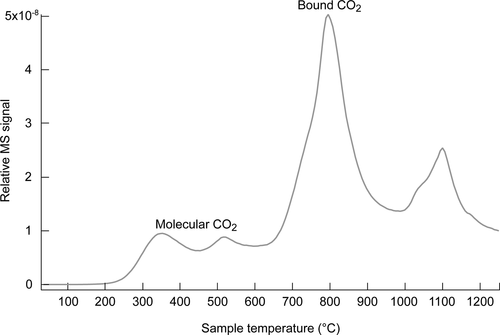
In this study, CO2 is equilibrated with molten sodium metasilicate (Na2SiO3). Dissolved CO2 is analyzed with postexposure carbon analyses and evolved gas analysis (EGA). We interpret these data together with literature data on similar systems. All systems show retrograde solubility, that is, higher solubility at lower temperatures. The evolved gas analysis suggests two forms of CO2 solubility: dissolved CO2 as a molecule to a limited extent and primarily CO2 bound as a compound, likely as Na2CO3. A comparison of heats of solution for this and other systems also suggests compound formation due to the larger heats of solution. The activities of Na2O and Na2CO3 are calculated for this Na2O–CO2–SiO2 system and extended to some sodium aluminosilicate melts as well. Such calculations allow determination of the miscibility limit for Na2CO3 in Na2SiO3 and factors which influence it.
中文翻译:

CO 2在硅酸钠熔体中的溶解度
在这项研究中,CO 2与熔融的偏硅酸钠(Na 2 SiO 3)平衡。用暴露后碳分析和逸出气体分析(EGA)分析溶解的CO 2。我们将这些数据与相似系统上的文献数据一起解释。所有系统均显示出逆行溶解度,即在较低温度下具有较高的溶解度。逸出的气体分析表明有两种形式的CO 2溶解度:溶解的CO 2作为分子在一定程度上有限,主要是作为化合物结合的CO 2,很可能是Na 2 CO 3。比较该系统和其他系统的溶液热还表明,由于溶液热较大,因此会形成化合物。Na 2 O– Na 2 O和Na 2 CO 3的活性是针对该Na 2 O–CO 2 –SiO 2系统计算的,并且还扩展到某些硅铝酸钠熔体。这样的计算允许确定Na 2 SiO 3中Na 2 CO 3的混溶性极限以及影响它的因素。
更新日期:2020-11-19
中文翻译:

CO 2在硅酸钠熔体中的溶解度
在这项研究中,CO 2与熔融的偏硅酸钠(Na 2 SiO 3)平衡。用暴露后碳分析和逸出气体分析(EGA)分析溶解的CO 2。我们将这些数据与相似系统上的文献数据一起解释。所有系统均显示出逆行溶解度,即在较低温度下具有较高的溶解度。逸出的气体分析表明有两种形式的CO 2溶解度:溶解的CO 2作为分子在一定程度上有限,主要是作为化合物结合的CO 2,很可能是Na 2 CO 3。比较该系统和其他系统的溶液热还表明,由于溶液热较大,因此会形成化合物。Na 2 O– Na 2 O和Na 2 CO 3的活性是针对该Na 2 O–CO 2 –SiO 2系统计算的,并且还扩展到某些硅铝酸钠熔体。这样的计算允许确定Na 2 SiO 3中Na 2 CO 3的混溶性极限以及影响它的因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号