当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Distinguishing and Stimulus-Responsive Carrier-Free Theranostic Nanoagents for Imaging-Guided Chemo-Photothermal Therapy in Small-Cell Lung Cancer
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2020-11-06 , DOI: 10.1021/acsami.0c18273 Qixin Zhu 1 , Zhongxiong Fan 1 , Wenbao Zuo 1 , Yilin Chen 1 , Zhenqing Hou 1 , Xuan Zhu 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2020-11-06 , DOI: 10.1021/acsami.0c18273 Qixin Zhu 1 , Zhongxiong Fan 1 , Wenbao Zuo 1 , Yilin Chen 1 , Zhenqing Hou 1 , Xuan Zhu 1
Affiliation
|
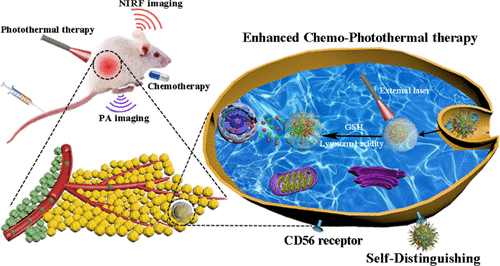
Lack of tumor targeting and low drug payload severely impedes various nanoagents further employed in small-cell lung cancer (SCLC). Therefore, how to develop a new targeting ligand and enhance drug payload has been an urgent need for SCLC therapy. Herein, we first sift and verify that capreomycin (Cm) has a high affinity toward CD56 receptors overexpressed on SCLC cells. Motivated by the concept of self-targeted drug delivery, Cm is selected as the specific targeting ligand toward CD56 receptors and chemodrug doxorubicin (Dox) is adopted to be covalently linked via the redox-responsive disulfide linkage. The synthesized self-distinguishing prodrug (Dox-ss-Cm) and FDA-approved photosensitizer indocyanine green (ICG) as structural motifs can be self-assembled into theranostic nanoagents (ICG@Dox-ss-Cm NPs) within an aqueous solution. Such carrier-free nanoagents with high drug payload can exert targeted on-demand drug release under multiple stimuli of intracellular lysosomal acidity, glutathione (GSH), and an external near-infrared (NIR) laser. Besides, our nanoagents can be specifically self-targeted to SCLC sites in vivo and self-distinguishing via SCLC cells in vitro; thus, they decrease the undesirable effects on normal tissues and organs. Further in vitro and in vivo studies uniformly confirm that such nanoagents show highly synergistic effects for SCLC chemo-photothermal therapy (PTT) under the precise guidance of NIR fluorescence (NIRF)/photoacoustic (PA) imaging. Taken together, our work can provide a novel and promising strategy for the targeted treatment of SCLC.
中文翻译:

自成像和无刺激响应的无载体治疗性纳米剂,用于小细胞肺癌的影像引导化学光热疗法
缺乏肿瘤靶向性和低药物有效载荷严重阻碍了进一步用于小细胞肺癌(SCLC)的各种纳米药物。因此,如何开发新的靶向配体和增加药物有效负载一直是SCLC治疗的迫切需要。在本文中,我们首先进行筛选并验证了霉素(Cm)对在SCLC细胞上过度表达的CD56受体具有高亲和力。出于自我靶向药物输送的概念,选择Cm作为针对CD56受体的特异性靶向配体,并采用化学药物阿霉素(Dox)通过氧化还原反应性二硫键。合成的自区分前药(Dox-ss-Cm)和FDA批准的光敏剂吲哚菁绿(ICG)作为结构基序可以在水溶液中自组装为诊断型纳米剂(ICG @ Dox-ss-Cm NPs)。具有高药物有效负载的此类无载体纳米剂可在细胞内溶酶体酸度,谷胱甘肽(GSH)和外部近红外(NIR)激光的多重刺激下,实现有针对性的按需药物释放。此外,我们的纳米剂可以在体内特异性地靶向SCLC部位,并在体外通过SCLC细胞进行自我区分。因此,它们减少了对正常组织和器官的不良影响。进一步的体外和体内研究一致证实,这种纳米药物在NIR荧光(NIRF)/光声(PA)成像的精确指导下,对SCLC化学光热疗法(PTT)表现出高度的协同作用。综上所述,我们的工作可以为SCLC的靶向治疗提供一种新颖而有希望的策略。
更新日期:2020-11-06
中文翻译:

自成像和无刺激响应的无载体治疗性纳米剂,用于小细胞肺癌的影像引导化学光热疗法
缺乏肿瘤靶向性和低药物有效载荷严重阻碍了进一步用于小细胞肺癌(SCLC)的各种纳米药物。因此,如何开发新的靶向配体和增加药物有效负载一直是SCLC治疗的迫切需要。在本文中,我们首先进行筛选并验证了霉素(Cm)对在SCLC细胞上过度表达的CD56受体具有高亲和力。出于自我靶向药物输送的概念,选择Cm作为针对CD56受体的特异性靶向配体,并采用化学药物阿霉素(Dox)通过氧化还原反应性二硫键。合成的自区分前药(Dox-ss-Cm)和FDA批准的光敏剂吲哚菁绿(ICG)作为结构基序可以在水溶液中自组装为诊断型纳米剂(ICG @ Dox-ss-Cm NPs)。具有高药物有效负载的此类无载体纳米剂可在细胞内溶酶体酸度,谷胱甘肽(GSH)和外部近红外(NIR)激光的多重刺激下,实现有针对性的按需药物释放。此外,我们的纳米剂可以在体内特异性地靶向SCLC部位,并在体外通过SCLC细胞进行自我区分。因此,它们减少了对正常组织和器官的不良影响。进一步的体外和体内研究一致证实,这种纳米药物在NIR荧光(NIRF)/光声(PA)成像的精确指导下,对SCLC化学光热疗法(PTT)表现出高度的协同作用。综上所述,我们的工作可以为SCLC的靶向治疗提供一种新颖而有希望的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号