当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational changes of the nucleotide binding domains of P‐glycoprotein induced by ATP hydrolysis
FEBS Letters ( IF 3.0 ) Pub Date : 2020-12-10 , DOI: 10.1002/1873-3468.13992 Sepehr Dehghani-Ghahnaviyeh 1 , Karan Kapoor 1 , Emad Tajkhorshid 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-12-10 , DOI: 10.1002/1873-3468.13992 Sepehr Dehghani-Ghahnaviyeh 1 , Karan Kapoor 1 , Emad Tajkhorshid 1
Affiliation
|
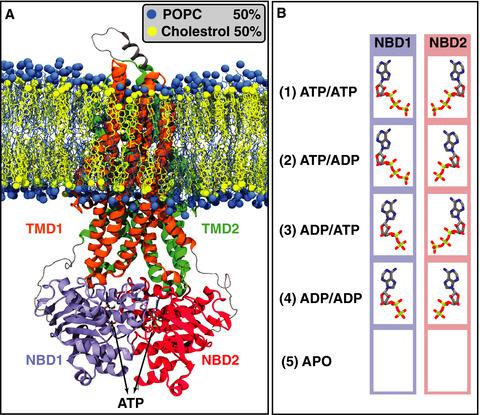
P-glycoprotein (Pgp) is a member of the ABC transporter superfamily with high physiological importance. Pgp nucleotide binding domains (NBDs) drive the transport cycle through ATP binding and hydrolysis. We use molecular dynamics simulations to investigate the ATP hydrolysis-induced conformational changes of NBDs. Five systems, including all possible ATP/ADP combinations in the NBDs and the APO system, are simulated. ATP/ADP exchange induces conformational changes mostly within the conserved signature motif of the NBDs, resulting in relative orientational changes of the NBDs. Nucleotide removal leads to additional orientational changes in the NBDs, allowing their dissociation. Furthermore, we capture putative hydrolysis-competent configurations in which the conserved glutamate in the WalkerB motif acts as a catalytic base capturing a water molecule likely initiating ATP hydrolysis.
中文翻译:

ATP水解诱导的P-糖蛋白核苷酸结合结构域的构象变化
P-糖蛋白 (Pgp) 是 ABC 转运蛋白超家族的成员,具有很高的生理意义。Pgp 核苷酸结合域 (NBD) 通过 ATP 结合和水解驱动运输循环。我们使用分子动力学模拟来研究 ATP 水解诱导的 NBDs 构象变化。模拟了五个系统,包括 NBD 和 APO 系统中所有可能的 ATP/ADP 组合。ATP/ADP 交换主要在 NBDs 的保守特征基序内诱导构象变化,导致 NBDs 的相对方向变化。核苷酸的去除导致 NBD 的额外方向变化,允许它们解离。此外,
更新日期:2020-12-10
中文翻译:

ATP水解诱导的P-糖蛋白核苷酸结合结构域的构象变化
P-糖蛋白 (Pgp) 是 ABC 转运蛋白超家族的成员,具有很高的生理意义。Pgp 核苷酸结合域 (NBD) 通过 ATP 结合和水解驱动运输循环。我们使用分子动力学模拟来研究 ATP 水解诱导的 NBDs 构象变化。模拟了五个系统,包括 NBD 和 APO 系统中所有可能的 ATP/ADP 组合。ATP/ADP 交换主要在 NBDs 的保守特征基序内诱导构象变化,导致 NBDs 的相对方向变化。核苷酸的去除导致 NBD 的额外方向变化,允许它们解离。此外,









































 京公网安备 11010802027423号
京公网安备 11010802027423号