当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elimination profiles of prednisone and prednisolone after different administration routes: Evaluation of the reporting level and washout periods to ensure safe therapeutic administrations
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-11-08 , DOI: 10.1002/dta.2966 Sergi Coll 1 , Núria Monfort 1 , Élida Alechaga 1 , Xavier Matabosch 1 , Oscar J Pozo 2 , Clara Pérez-Mañá 3, 4 , Rosa Ventura 1, 5
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-11-08 , DOI: 10.1002/dta.2966 Sergi Coll 1 , Núria Monfort 1 , Élida Alechaga 1 , Xavier Matabosch 1 , Oscar J Pozo 2 , Clara Pérez-Mañá 3, 4 , Rosa Ventura 1, 5
Affiliation
|
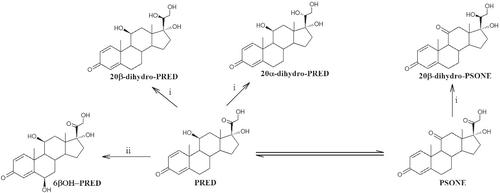
Prednisolone (PRED) and prednisone (PSONE) are prohibited in sports competitions when administered by systemic routes, and they are allowed by other routes for therapeutic purposes. There is no restriction of use in out‐of‐competition periods. The present study aimed to evaluate the urinary excretion of PRED, PSONE, and their most important metabolites after systemic and nonsystemic treatments in order to verify the suitability of the current reporting level of 30 ng/ml used to distinguish allowed and prohibited administrations and to establish washout periods for oral treatments performed in out‐of‐competition periods. PRED was studied after dermatological administration (5 mg/day for 5 days, n = 6 males) and oral administration (5 mg, n = 6 males; 10 mg, n = 2 males). PSONE was studied after oral administration (10 mg, n = 2 males; 30 mg, n = 1 male and 1 female). Concentrations in urine were measured using an LC–MS/MS method. Concentrations after dermatological treatment were low for all metabolites. After oral administration, concentrations were very high during the first 24 h after administration ranging from 1.6 to 2261 ng/ml and from 4.6 to 908 ng/ml for PRED and PSONE, respectively. Concentrations of most of the metabolites measured were lower than 30 ng/ml from 24 h after all oral administrations. New reporting levels are proposed for PRED and PSONE considering data of our study and other information published after nonsystemic administrations of the compounds. Washout periods of at least 24 h are recommended to ensure no false positives when oral treatments need to be performed in out‐of‐competition periods.
中文翻译:

不同给药途径后泼尼松和泼尼松龙的消除概况:评估报告水平和清除期以确保安全的治疗给药
体育比赛中禁止通过全身给药途径使用泼尼松龙 (PRED) 和泼尼松 (PSONE),出于治疗目的允许通过其他途径给药。在赛外期间没有使用限制。本研究旨在评估全身和非全身治疗后 PRED、PSONE 及其最重要代谢物的尿排泄,以验证当前报告水平 30 ng/ml 的适用性,用于区分允许和禁止给药,并确定在赛外期间进行的口服治疗的洗脱期。在皮肤科给药(5 毫克/天,连续 5 天,n = 6 名男性)和口服给药(5 毫克,n = 6 名男性;10 毫克,n= 2 名男性)。口服给药后研究了 PSONE(10 毫克,n = 2 名男性;30 毫克,n= 1 男 1 女)。使用 LC-MS/MS 方法测量尿液中的浓度。皮肤病治疗后所有代谢物的浓度都很低。在口服给药后的第一个 24 小时内,PRED 和 PSONE 的浓度分别为 1.6 至 2261 ng/ml 和 4.6 至 908 ng/ml。在所有口服给药后 24 小时内,测量的大多数代谢物的浓度低于 30 ng/ml。考虑到我们的研究数据和非全身给药后公布的其他信息,为 PRED 和 PSONE 提出了新的报告水平。建议至少 24 小时的冲洗期,以确保在赛外期间需要进行口服治疗时不会出现假阳性。
更新日期:2020-11-08
中文翻译:

不同给药途径后泼尼松和泼尼松龙的消除概况:评估报告水平和清除期以确保安全的治疗给药
体育比赛中禁止通过全身给药途径使用泼尼松龙 (PRED) 和泼尼松 (PSONE),出于治疗目的允许通过其他途径给药。在赛外期间没有使用限制。本研究旨在评估全身和非全身治疗后 PRED、PSONE 及其最重要代谢物的尿排泄,以验证当前报告水平 30 ng/ml 的适用性,用于区分允许和禁止给药,并确定在赛外期间进行的口服治疗的洗脱期。在皮肤科给药(5 毫克/天,连续 5 天,n = 6 名男性)和口服给药(5 毫克,n = 6 名男性;10 毫克,n= 2 名男性)。口服给药后研究了 PSONE(10 毫克,n = 2 名男性;30 毫克,n= 1 男 1 女)。使用 LC-MS/MS 方法测量尿液中的浓度。皮肤病治疗后所有代谢物的浓度都很低。在口服给药后的第一个 24 小时内,PRED 和 PSONE 的浓度分别为 1.6 至 2261 ng/ml 和 4.6 至 908 ng/ml。在所有口服给药后 24 小时内,测量的大多数代谢物的浓度低于 30 ng/ml。考虑到我们的研究数据和非全身给药后公布的其他信息,为 PRED 和 PSONE 提出了新的报告水平。建议至少 24 小时的冲洗期,以确保在赛外期间需要进行口服治疗时不会出现假阳性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号