当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
KLiSiF6 and CsLiSiF6 – A Structure Investigation
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-11-06 , DOI: 10.1002/ejic.202000867 Christiane Stoll 1 , Markus Seibald 2 , Dominik Baumann 2 , Jascha Bandemehr 3 , Florian Kraus 3 , Hubert Huppertz 1
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-11-06 , DOI: 10.1002/ejic.202000867 Christiane Stoll 1 , Markus Seibald 2 , Dominik Baumann 2 , Jascha Bandemehr 3 , Florian Kraus 3 , Hubert Huppertz 1
Affiliation
|
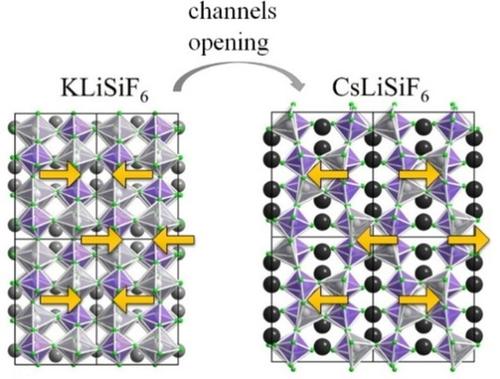
KLiSiF6 and CsLiSiF6 were synthesized via a high‐pressure/high‐temperature synthesis route. Even though, both substances crystallize in the orthorhombic crystal system with space group Pbcn (no. 60), they show different crystal structures. Main motifs of KLiSiF6 are an [SiF6]2− and an [LiF6]5− unit. In contrast, the main motifs of CsLiSiF6 are an [SiF6]2− and an [LiF5]4− entity. Within both substances, these units are interconnected and form 3‐dimensional networks. The substances were characterized via single‐crystal and powder X‐ray diffraction, as well as infrared spectroscopy and EDX measurements. Additionally, Mn4+‐doped KLiSiF6 was analyzed by means of luminescence spectroscopy. It displays line emission in the red spectral region. The maximum emission wavelength is λmax=631 nm.
中文翻译:

KLiSiF6和CsLiSiF6 –结构研究
KLiSiF 6和CsLiSiF 6合成通过高压/高温的合成路线。即使这两种物质都在具有空间群Pbcn(第60号)的正交晶体系统中结晶,但它们显示出不同的晶体结构。KLiSiF 6的主要基序是[SiF 6 ] 2−和[LiF 6 ] 5−单元。相反,CsLiSiF 6的主要基序是[SiF 6 ] 2−和[LiF 5 ] 4−实体。在这两种物质中,这些单元相互连接并形成三维网络。通过单晶和粉末X射线衍射,红外光谱和EDX测量对这些物质进行表征。此外,还通过发光光谱法分析了掺杂Mn 4+的KLiSiF 6。它在红色光谱区域显示线发射。最大发射波长是λmax = 631nm。
更新日期:2021-01-05
中文翻译:

KLiSiF6和CsLiSiF6 –结构研究
KLiSiF 6和CsLiSiF 6合成通过高压/高温的合成路线。即使这两种物质都在具有空间群Pbcn(第60号)的正交晶体系统中结晶,但它们显示出不同的晶体结构。KLiSiF 6的主要基序是[SiF 6 ] 2−和[LiF 6 ] 5−单元。相反,CsLiSiF 6的主要基序是[SiF 6 ] 2−和[LiF 5 ] 4−实体。在这两种物质中,这些单元相互连接并形成三维网络。通过单晶和粉末X射线衍射,红外光谱和EDX测量对这些物质进行表征。此外,还通过发光光谱法分析了掺杂Mn 4+的KLiSiF 6。它在红色光谱区域显示线发射。最大发射波长是λmax = 631nm。











































 京公网安备 11010802027423号
京公网安备 11010802027423号