当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From Synthetic to Biological Fe4S4 Complexes: Redox Properties Correlated to Function of Radical S‐Adenosylmethionine Enzymes
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-11-09 , DOI: 10.1002/cplu.202000663 Daniel Bím 1 , Santiago Alonso-Gil 1 , Martin Srnec 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-11-09 , DOI: 10.1002/cplu.202000663 Daniel Bím 1 , Santiago Alonso-Gil 1 , Martin Srnec 1
Affiliation
|
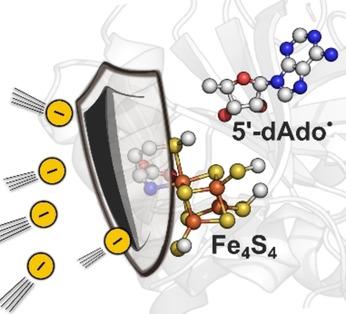
By employing the computational protocol for calculation of reduction potentials of the Fe4S4‐containing species validated using a representative series of well‐defined synthetic complexes, we focused on redox properties of two prototypical radical SAM enzymes to reveal how they transform SAM into the reactive 5’‐deoxyadenosyl radical, and how they tune this radical for its proper biological function. We found the reduction potential of SAM is indeed elevated by 0.3–0.4 V upon coordination to Fe4S4, which was previously speculated in the literature. This makes a generation of 5’‐deoxyadenosyl radical from SAM less endergonic (by ca. 7–9 kcal mol−1) and hence more feasible in both enzymes as compared to the identical process in water. Furthermore, our calculations indicate that the enzyme‐bound 5’‐deoxyadenosyl radical has a significantly lower reduction potential than in referential aqueous solution, which may help the enzymes to suppress potential side redox reactions and simultaneously elevate its proton‐philic character, which may, in turn, promote the radical hydrogen‐atom abstraction ability.
中文翻译:

从合成到生物 Fe4S4 配合物:与自由基 S-腺苷甲硫氨酸酶功能相关的氧化还原特性
通过使用计算协议来计算含Fe 4 S 4物种的还原电位,使用一系列具有代表性的定义明确的合成配合物进行验证,我们专注于两种原型自由基 SAM 酶的氧化还原特性,以揭示它们如何将 SAM 转化为反应性 5'-脱氧腺苷自由基,以及他们如何调整该自由基以使其具有适当的生物学功能。我们发现 SAM 的还原电位在与 Fe 4 S 4配位后确实提高了 0.3-0.4 V ,这在之前的文献中是推测的。这使得来自 SAM 的 5'-脱氧腺苷自由基的能量降低(约 7-9 kcal mol -1),因此与在水中的相同过程相比,在两种酶中更可行。此外,我们的计算表明,与参考水溶液相比,酶结合的 5'-脱氧腺苷自由基具有显着较低的还原电位,这可能有助于酶抑制潜在的副氧化还原反应,同时提高其亲质子特性,这可能,反过来,促进自由基提取氢原子的能力。
更新日期:2020-11-27
中文翻译:

从合成到生物 Fe4S4 配合物:与自由基 S-腺苷甲硫氨酸酶功能相关的氧化还原特性
通过使用计算协议来计算含Fe 4 S 4物种的还原电位,使用一系列具有代表性的定义明确的合成配合物进行验证,我们专注于两种原型自由基 SAM 酶的氧化还原特性,以揭示它们如何将 SAM 转化为反应性 5'-脱氧腺苷自由基,以及他们如何调整该自由基以使其具有适当的生物学功能。我们发现 SAM 的还原电位在与 Fe 4 S 4配位后确实提高了 0.3-0.4 V ,这在之前的文献中是推测的。这使得来自 SAM 的 5'-脱氧腺苷自由基的能量降低(约 7-9 kcal mol -1),因此与在水中的相同过程相比,在两种酶中更可行。此外,我们的计算表明,与参考水溶液相比,酶结合的 5'-脱氧腺苷自由基具有显着较低的还原电位,这可能有助于酶抑制潜在的副氧化还原反应,同时提高其亲质子特性,这可能,反过来,促进自由基提取氢原子的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号