当前位置:
X-MOL 学术
›
Mater. Today Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly efficient, economic, and recyclable glutathione decorated magnetically separable nanocomposite for uranium(VI) adsorption from aqueous solution
Materials Today Chemistry ( IF 6.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.mtchem.2020.100379 M. Sharma , K. Chaudhary , M. Kumari , P. Yadav , K. Sachdev , V. Chandra Janu , R. Gupta
Materials Today Chemistry ( IF 6.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.mtchem.2020.100379 M. Sharma , K. Chaudhary , M. Kumari , P. Yadav , K. Sachdev , V. Chandra Janu , R. Gupta
|
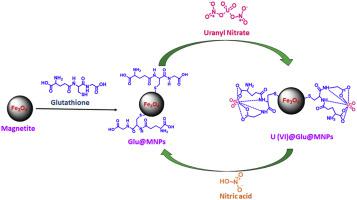
Abstract A green and environment-friendly magnetically separable nanocomposite, glutathione@magnetite was fabricated sonochemically through the functionalization of Fe3O4 by glutathione which was well characterized using Fourier-transform infrared spectroscopy, ultravoilet-visible spectroscopy, scanning electron microscope, energy-dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, thermogravimetric analysis, vibrating sample magnetometer, Brunauer-Emmett-Teller, and high-resolution transmission electron microscope. The parameters affecting adsorption including pH, temperature, contact time, initial adsorbate concentration, and adsorbent amount were optimized by batch experiments. The magnetic glutathione@magnetite was applied for the removal of uranium(VI) in water with maximum adsorption capacity found to be 333.33 mg/g in 120 min at a neutral pH at 25 °C showing high efficiency for U(VI) ions. Furthermore, adsorption results obtained from UV-vis spectroscopy were validated by inductively coupled plasma optical emission spectroscopy. The thermodynamic parameters, viz Gibbs free energy (ΔGo), standard enthalpy change (ΔHo), and standard entropy change (ΔSo) of the process were calculated using the Langmuir constants. The pseudo-second-order kinetics model is seen to be applicable for describing the uptake process using a kinetics test. Moreover, desorption studies reveals that glutathione@magnetite can be used repeatedly, and removal efficiency shows only a small decrease after six cycles. Thus, glutathione@magnetite acts as a potential adsorbent for the removal of U(VI) from the water with great adsorption performance.
中文翻译:

高效、经济、可回收的谷胱甘肽装饰磁分离纳米复合材料,用于从水溶液中吸附铀(VI)
摘要 谷胱甘肽对 Fe3O4 进行功能化,通过声化学方法制备了一种绿色环保的磁性可分离纳米复合材料 glutathione@magnetite,利用傅里叶变换红外光谱、紫外可见光谱、扫描电子显微镜、能量色散 X 射线对其进行了表征。光谱、X 射线光电子能谱、X 射线衍射、热重分析、振动样品磁强计、Brunauer-Emmett-Teller 和高分辨率透射电子显微镜。通过批量实验对影响吸附的参数包括pH、温度、接触时间、初始吸附物浓度和吸附剂用量进行了优化。磁性谷胱甘肽@磁铁矿用于去除水中的铀 (VI),发现最大吸附容量为 333。在 25 °C 的中性 pH 值下,120 分钟内为 33 mg/g,显示出对 U(VI) 离子的高效率。此外,从紫外-可见光谱获得的吸附结果通过电感耦合等离子体发射光谱进行了验证。使用朗缪尔常数计算该过程的热力学参数,即吉布斯自由能 (ΔGo)、标准焓变 (ΔHo) 和标准熵变 (ΔSo)。伪二级动力学模型被认为适用于使用动力学测试描述吸收过程。此外,解吸研究表明,谷胱甘肽@磁铁矿可以重复使用,六次循环后去除效率仅略有下降。因此,谷胱甘肽@磁铁矿作为一种潜在的吸附剂,可以从水中去除 U(VI),具有很好的吸附性能。
更新日期:2020-12-01
中文翻译:

高效、经济、可回收的谷胱甘肽装饰磁分离纳米复合材料,用于从水溶液中吸附铀(VI)
摘要 谷胱甘肽对 Fe3O4 进行功能化,通过声化学方法制备了一种绿色环保的磁性可分离纳米复合材料 glutathione@magnetite,利用傅里叶变换红外光谱、紫外可见光谱、扫描电子显微镜、能量色散 X 射线对其进行了表征。光谱、X 射线光电子能谱、X 射线衍射、热重分析、振动样品磁强计、Brunauer-Emmett-Teller 和高分辨率透射电子显微镜。通过批量实验对影响吸附的参数包括pH、温度、接触时间、初始吸附物浓度和吸附剂用量进行了优化。磁性谷胱甘肽@磁铁矿用于去除水中的铀 (VI),发现最大吸附容量为 333。在 25 °C 的中性 pH 值下,120 分钟内为 33 mg/g,显示出对 U(VI) 离子的高效率。此外,从紫外-可见光谱获得的吸附结果通过电感耦合等离子体发射光谱进行了验证。使用朗缪尔常数计算该过程的热力学参数,即吉布斯自由能 (ΔGo)、标准焓变 (ΔHo) 和标准熵变 (ΔSo)。伪二级动力学模型被认为适用于使用动力学测试描述吸收过程。此外,解吸研究表明,谷胱甘肽@磁铁矿可以重复使用,六次循环后去除效率仅略有下降。因此,谷胱甘肽@磁铁矿作为一种潜在的吸附剂,可以从水中去除 U(VI),具有很好的吸附性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号